JMLS 2016 April;1(1):70-78. Epub 2016 April 30
Copyright © 2016 by The Korean Society of Marine Life Science
Current Advances in Cryopreservation of Microalgae
Wahyu Sri Kunto Nugroho; Department of Chemistry, Pukyong National University, Busan 48513, Korea
Do-A Kim; Department of Chemistry, Pukyong National University, Busan 48513, Korea
Dong-Woo Kim; Department of Chemistry, Pukyong National University, Busan 48513, Korea
Bon-Won Koo; Department of Chemistry, Pukyong National University, Busan 48513, Korea
Young Baek Hur; Southeast Sea Fisheries Research Institute, National Fisheries Research and Development Institute, Namhae 52440, Korea
Hak Jun Kim; Department of Chemistry, Pukyong National University, Busan 48513, Korea
- Abstract
Microalgae are of significant importance for future biotechnological applications. Many microalgae banks or laboratories attempt to maintain various microalgae for further research purposes. Cryopreservation has been preferred to reduce a labor-intensive and costly routine sub-culturing. Cryopreservation can also diminish the genetic drift risk. However, cryopreservation as a long term storage of microalgae method are still in developing progress because it cannot be generalized for all microalgae. Microalgae types, cryoprotectant agents (CPAs) types, freezing and thawing methods are the most important factors that should be considered for cryopreservation. In this short review the basic principles and the current advanced of microalgae cryopreservation methods are discussed with a suggested starting parameters for microalgae cryopreservation.
Keywords: Microalgae Cryopreservation Cryoprtectants Intracellular ice formation
Correspondence to: Hak Jun Kim; Department of Chemistry, Pukyong National University, Busan 48513, Korea ,E-mail : kimhj@pknu.ac.kr
- Received
- 5 April 2016;
- Revised
- 7 April 2016;
- Accepted
- 25 April 2016.
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
1. Introduction
Microalgae are the fastest-growing plants that can be found in the freshwater and marine environments (Demirbas and Fatih Demirbas, 2011). Their biodiversity has been estimated more than 30,000 species (Guiry, 2012). This huge number represents a rich genetic resource and interest in their biotechnology potential. They draw an interest from biotechnology, especially thanks to their secondary metabolites as potential compounds such as antiinflammatory,antimicrobial, and antioxidant activities as well as biofuel (Rhodes et al., 2006; de Morais et al., 2015). To take the advantage of their potential, useful marine microalgae strains should be maintained for further research (Brand and Diller, 2004; de Morais et al., 2015).
Historically, microalgae collections have been maintained by a successive culturing (routine serial subculture) (Pringsheim, 1946; Day et al., 1997; Day et al., 2005). This method is preferred for the most phycologists when the number of strains are small. However, this method becomes problematic when a large number of microalgae (Day et al., 1997; Day et al., 2000). This approach needs huge space, growth media, labor cost, and etc. Therefore, maintaining a large number of microalgae in a collection using this method is costly (Borowitzka, 1997). In addition, there is a continuous risk of contamination and genetic drift occurring in serial sub-culturing (Rhodes et al., 2006; Heesch et al., 2012).
One of the preferred solution to this is cryopreservation (Polges et al., 1949). The main aim of cryopreservation is to prevent the transformation of morphological, biochemical, and hereditary properties (Polges et al., 1949; Day et al., 2000). In principle metabolism of the organism would be halted at a very low temperature, and be reactivated by thawing them. However, cryopreservation method available to date are not suitable to preserve all types of microalgae because each of microalgae has own unique properties (Abreu et al., 2012).
Many parameters should be considered to optimize cryopreservation. Here, we delineated the state of the art and current cryopreservation method for various types of microalgae including freezing and thawing methods, and cryoprotectants (CPAs).
2. Current Cryopreservation Technology
Cryopreservation has been proven able to maintain genotype of cryopreserved cells (Hipkin et al., 2013). Cryopreservation also has been successfully maintained the chlorophyll content of microalgae for 15 years (Nakanishi et al., 2012). Currently, cryopreservation method is a preferred alternative method for maintaining a large number of microalgae. However, the established method for cryopreservation is still limited to certain species.
The research to optimize the cryopreservation of microalgae has been grown and became an interesting research field.
Cryopreservation of microalgae uses two basic concepts: dehydration of cells using compatible solutes and the consequent prevention of intracellular ice crystal formation (Taylor and Fletcher, 1999). The dehydration of microalgae is used to prevent the ice growth in the inside microalgae. The intracellular ice growth can disrupt the microalgae.
Successful microalgae cryopreservation is generally assessed by viability levels, and genetic integrity. According to Day et al. (2005), good quality of cryopreservation means at least 10~60% post-thaw viability of the cryopreserved microalgae (Day et al.,2005). However, the most important thing to consider is the postthawed growth of microalgae without genetic changes.
The cryopreservation parameters such as microalgae cell concentration, culture age, centrifugation, freezing step rate, CPAs types and CPAs concentration have been examined and would be discussed here.
2.1. Cell concentration, culture age, and centrifugation
The first optimization step of microalgae cryopreservation is the cell concentration. High cell concentration has been reported to reduce the viability of microalgae. An injurious substance is enzymatically released upon microalgae death (Piasecki et al.,2002; Piasecki et al., 2008). Therefore, lower cell concentration seems to be beneficial (Bui et al., 2013). However, if the microalgae concentration is too low, it could reduce the chance of microalgae survival after cryopreservation. Generally, 1 × 106 cell/ml concentration is a good starting point for microalgae cryopreservation.
Microalgae culture age is also generally thought to affect viability level of cryopreserved samples (Cañavate and Lubián, 1997). The cultures age of mid and late exponential phase from 7 to 14 days are generally resulting high viability levels ±67% (McLellan, 1989; Harding et al., 2004). However, if the culture is in the late stationary phase, it would be in the death phase soon then the viability level is reduced (Lavens and Sorgeloos, 1996). Therefore, the growth rate of each strain should be determined before cyropreservation.
In the thawing step, cryoprotentants (CPAs) are usually removed to minimize the cytotoxic effect by centrifugation and washing multiple times during thawing step. However, according to Bui et al. (2013), the centrifugation step could decrease 22% of cell viability of post-thawed samples. Furthermore, this effect could be augmented by higher speed centrifugation. Shear forces between microalgae and centrifugation tube can damage algae cells, especially when they are fragile (post-thawed samples). Interestingly Youn and Hur (2009) showed that viability and growth of the microalgae were not influenced by the removal of CPAs after thawing. Hence, post-thaw centrifugation step is not suggested or recommended only using lower speed centrifugation (Bui et al., 2013).
2.2. Freezing rate
Cryopreservation is usually maintained at -196℃ (in liquid nitrogen) without serious damage (McLellan, 1989; Zhou et al.,2007). In order to reach this temperature, the freezing rate is controlled to reduce the formation of ice during freezing, which can be causing damage to the microalgae. Serious damage on the cryopreserved samples would lead to decrease of viability or death of microalgae in the post-thaw step. Generally, there are two types of freezing steps utilized: single step (rapid freezing), and two step (slow freezing at certain speed and then rapid freezing). Additionally, there is a vitrification method in which water molecules solidifies amorphously.
2.2.1. Single step freezing
In rapid single step freezing the microalgae samples in cryovial are directly plunged into liquid nitrogen (-196℃). Cells will be frozen rapidly in both extracellularly and intracellularly. The fatal intracellular ice formation should be prevented (Barry J. Fuller et al., 2004). In rapid freezing there is no time for water loss (dehydration) from the cell to minimize intracellular ice formation, resulting in lower viability. Therefore the single step rapid freezing is not recommended. However, some microalgae like diatom Navicula glaciei was shown to tolerate with single step freezing. This tolerance may be due to an antifreeze protein (AFP), icecrystal growth inhibitory protein that N. glaciei produces (Kang and Raymond, 2004). To date, AFPs have been extensively tested as a CPA. Some microalgae species with thick cell wall were reported to survive after single step freezing: Gloeocystis gigas, Nannochloris oculata, Stichococcus bacillaris, N. oceanica, N. salina, Nannochloris sp., Chrococcus minutus, Oscillatoria angustissima, Synechococcus nidulans, Trichodismium erythraeum (Youn and Hur, 2009).
2.2.2. Two step freezing
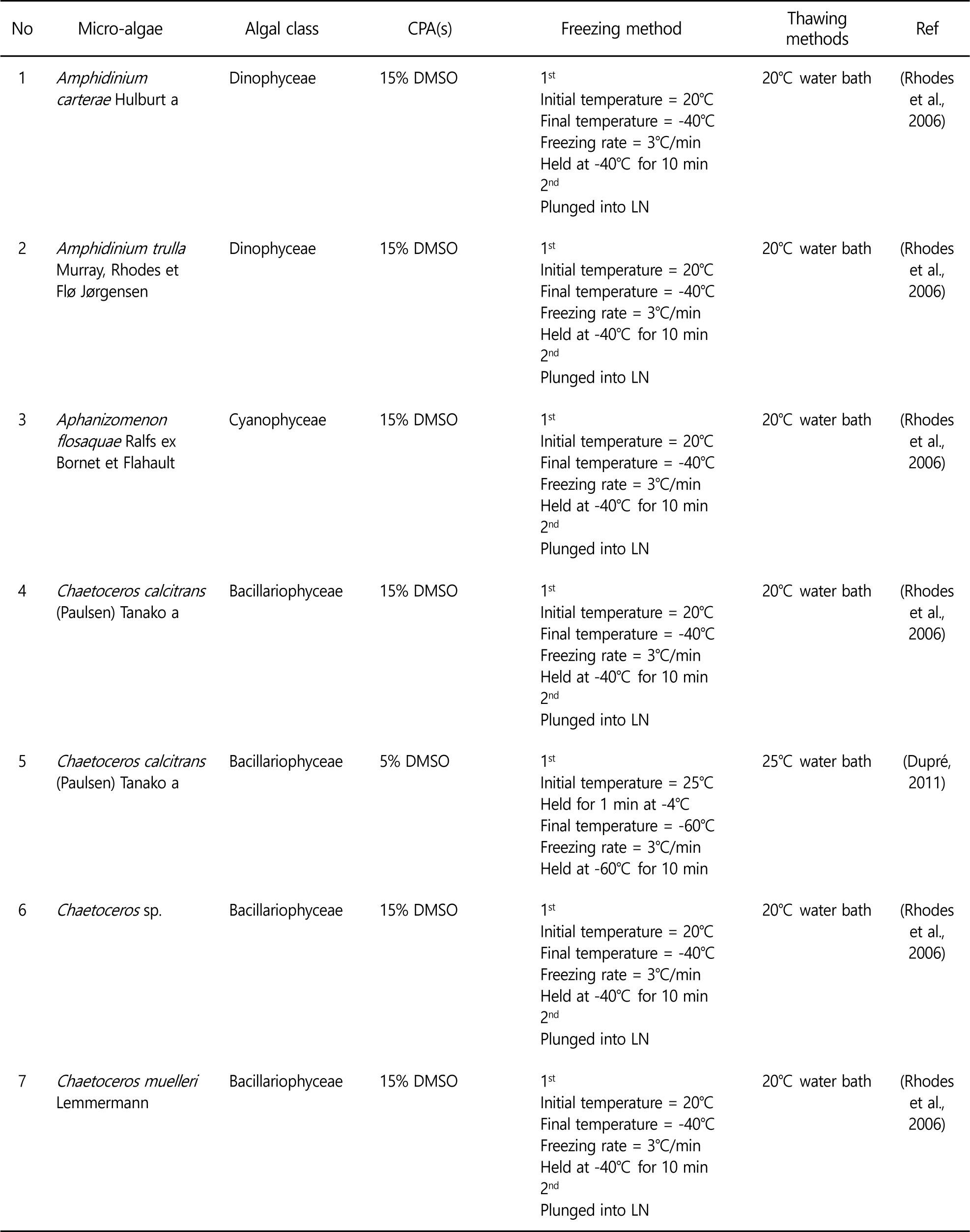
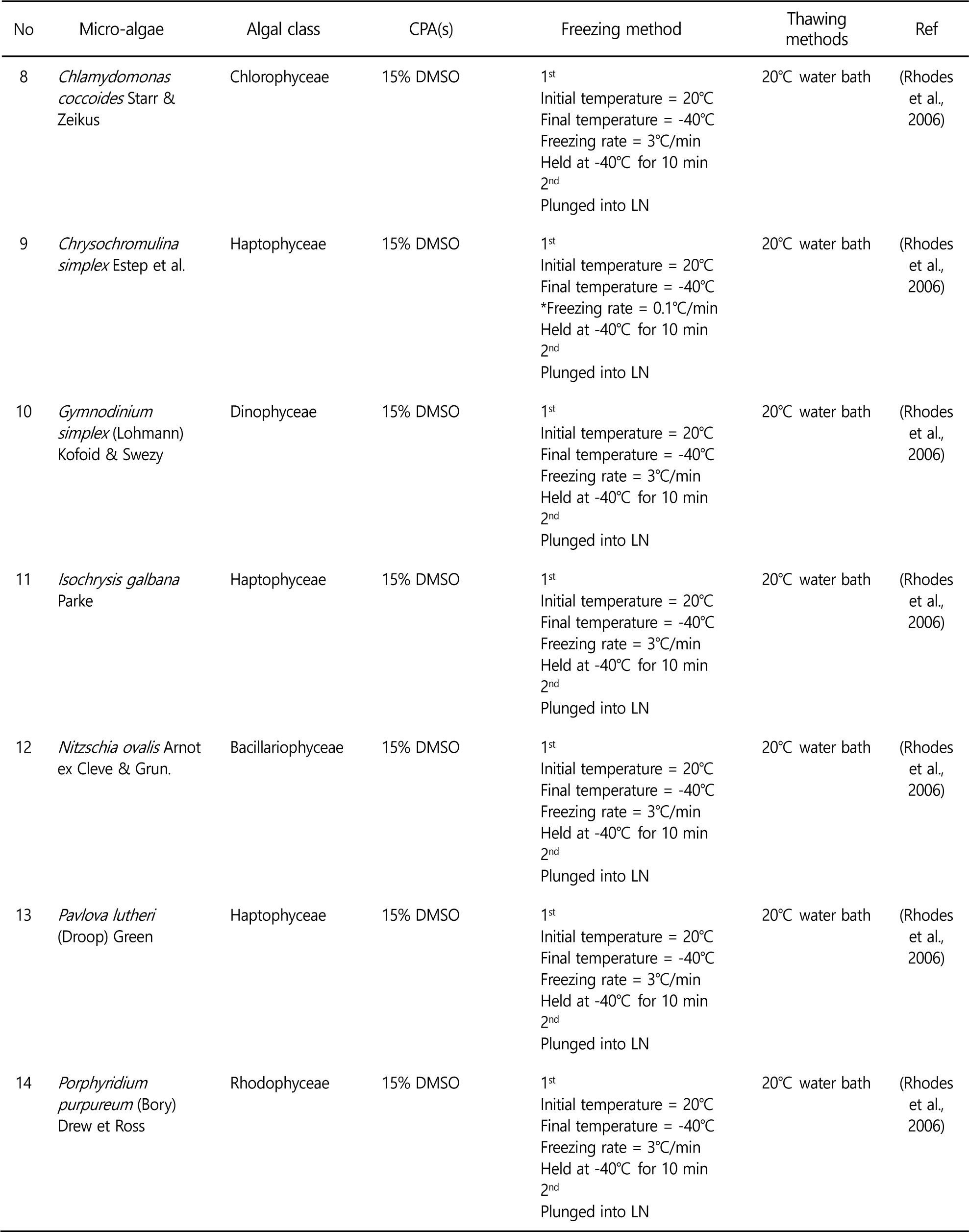
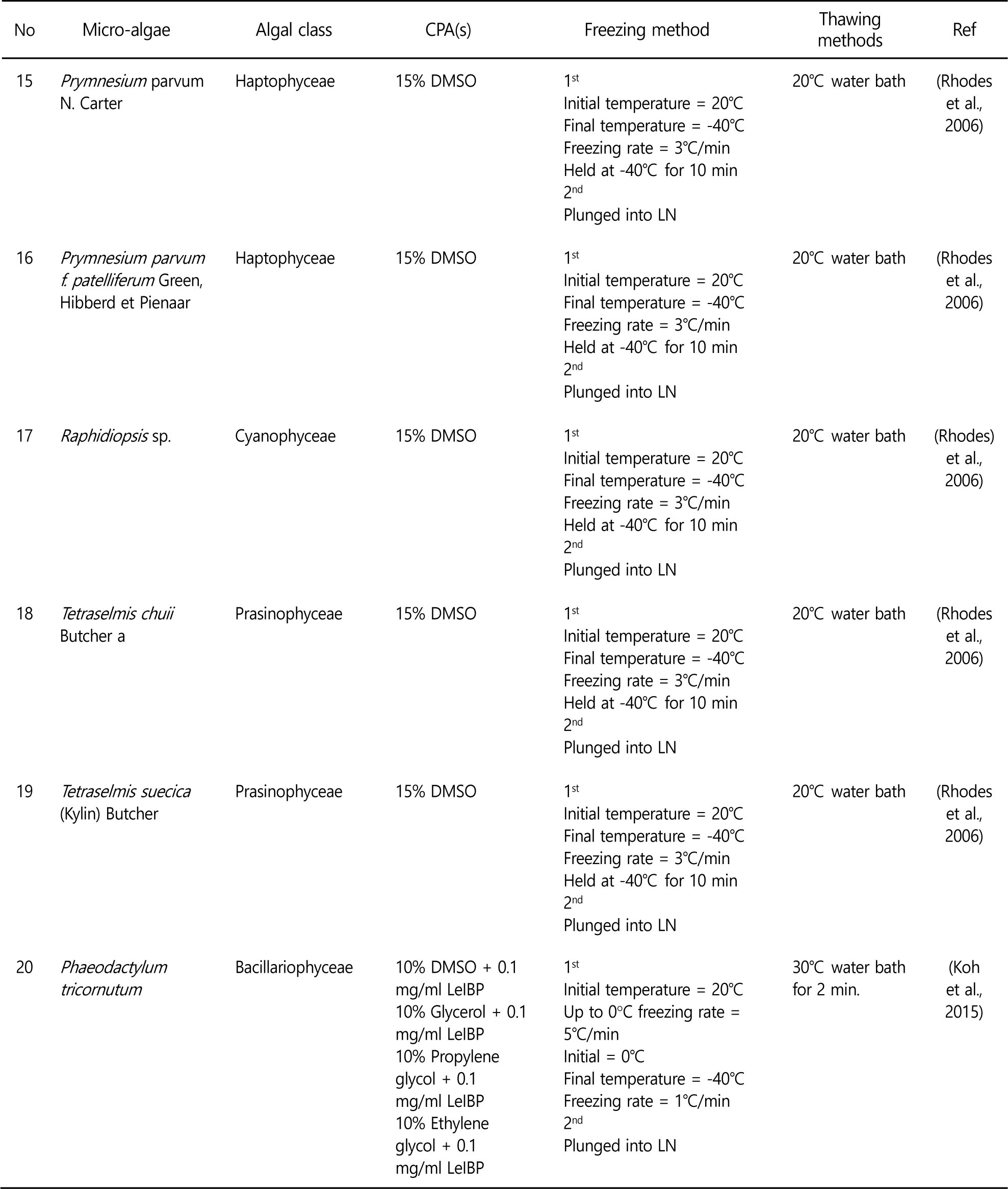
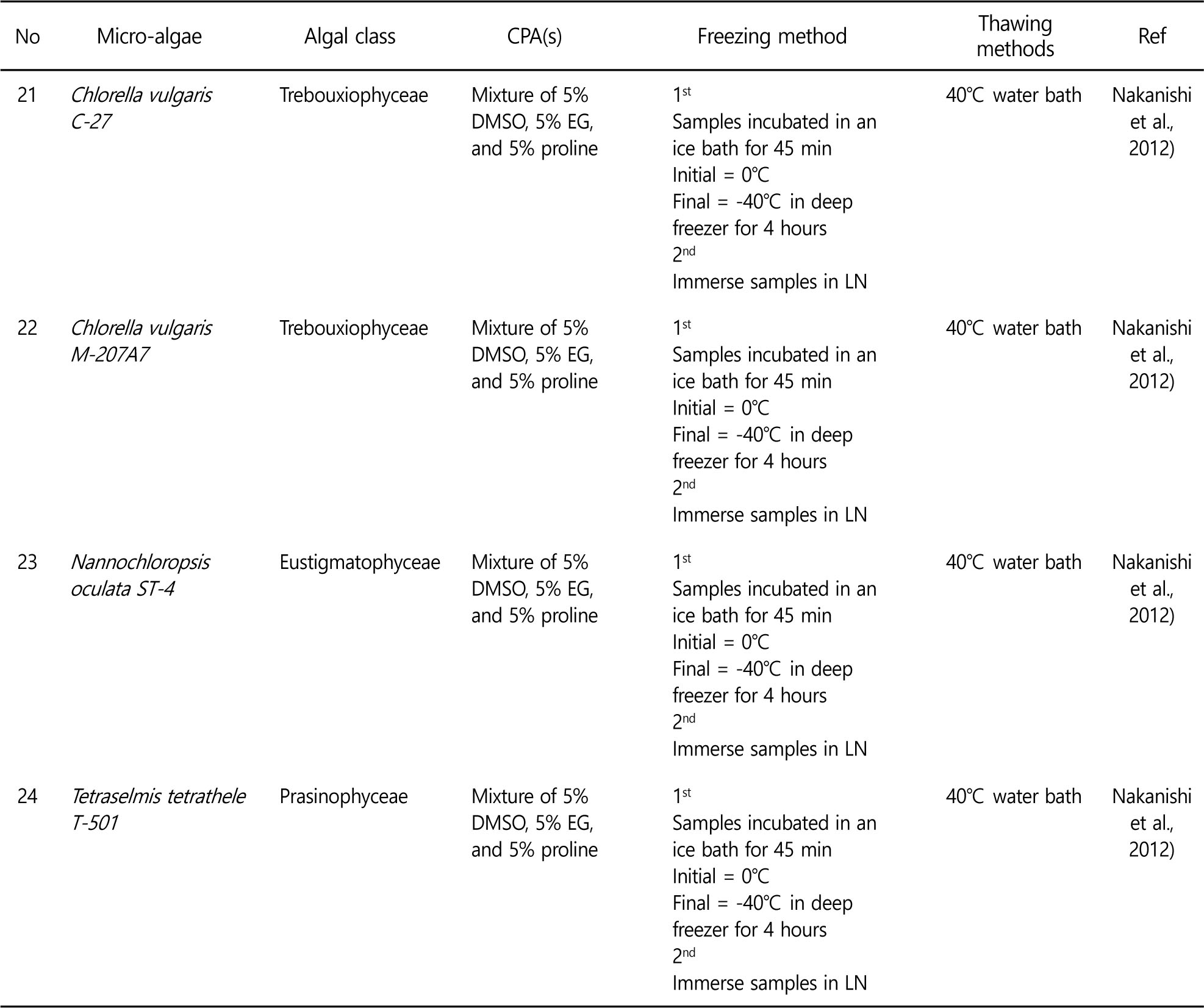
Controlling the freezing rates to avoid lethal intracellular ice formation is a key to successful cryopreservation. In the two step freezing, the microalgae samples are cooled to certain temperature at freezing rate, and then the samples are plunged into liquid nitrogen. Controlled rate freezing allows dehydration of microalgae. As a results, intracellular ice formation is minimized and higher viability levels are obtained. Therefore, two step freezing method is preferred in the microalgae cryopreservation. Some of successful microalgae cryopreservation are summarized in the Table 1. Various experiments to optimize cryopreservation of microalgae have been conducted because cryopreservation methods cannot be generalized and applied to all kinds of microalgae.
2.2.3. Vitrification
Vitrification is the transformation of liquid water into glassy water, where water still possesses a random molecular structure (amorphous) in solid state (Harding et al., 2004). Vitrification has been pioneered by Fahy et al. (1986) in medical cryobiology. In contrast with the two step freezing cryopreservation, the vitrification uses high cooling and warming rates to avoid ice crystal formation inside cells (Sibinga et al., 1986). Glasses are considered metastable, so it can easily form a solid crystalline during cryopreservation or thawing which can damage cells. Appropriate cooling and warming rates, important keys of vitrification and devitrification, should be determined for each species (Harding et al., 2004).

2.3. Cryoprotectants (CPAs)
Cryoprotectants are substances which can be used to reduce cryodamage (van der Meer and Simpson, 1984; Iwamoto et al.,2012). Generally, CPAs can be classified based on their ability to penetrate or nonpenetrate into cells. Penetrating CPAs can be equilibrated inside and outside solution of cells whereas nonpenetrating CPAs do not pass through the membrane or cell walls and remains extracellularly. Lower molecular weight is better than higher molecular weight in its penetrating ability. However, the penetration is highly dependent on the temperature and cell type (Hubálek, 2003).
2.3.1. Penetrating CPAs
In 1949, glycerol is used for the first time as a penetrating CPA which was used in cryopreservation (Polges et al., 1949). After that, other CPAs such as dimethylsulfoxide (DMSO), ethylene glycol, propylene glycol were examined in cryopreservation of many cells (Benson, 2008). Generally DMSO, methanol, ethylene glycol, propylene glycol, glycerol, polyethylene glycol, and PVP (polyvinylpyrrolidone) are CPAs used widely with acceptable results for microalgae cryopreservation (Polges et al., 1949). Penetrating CPAs are known to be more effective than nonpenetrating CPAs. However, some CPAs were discovered to be toxic at certain concentrations because microalgae susceptibility to the CPAs.
CPA toxicity is always the first priority to consider before cryopreservation of microalgae. If penetrating CPAs are used, it is advised to incubate the samples for a few minutes (15~30 minutes) before cryopreservation to allow the equilibrium of CPAs and microalgae (Lovelock, 1954). It should be noted that every microalgae also has its own permeability and susceptibility which can affect to the equilibration time and CPAs concentration (Tanaka et al., 2001). Consequently, the optimal type and concentration of CPA or combinations of CPAs, and equilibrium times should be determined empirically to increase the viability level of cryopreserved microalgae (Hubálek, 2003). These general CPAs propertieswhich commonly used for microalgae cryopreservation
are shown in the Table 2.
2.3.2. Nonpenetrating CPAs
Nonpenetrating CPAs means the CPAs are remained outside the microalgae cells. The advantage of nonpenetrating CPAs are much less toxic than the penetrating counterparts. Unlike penetrating CPAs, equilibration time is not needed if nonpenetrating CPAs are used. Nonpenetrating CPAs are not used widely for microalgae cryopreservation because they are less effective than penetrating CPAs. Therefore, the combination of penetrating and nonpenetrating CPAs are used to increase the viability levels. Recently, AFP or ice-binding proteins, ice growth inhibitor, were successfully used to improve the cryopreservation of microalgae Phaeodactylum tricornutum (Koh et al., 2015). There is still limited report about the AFPs as a CPAs additive in microalgae cryopreservation. As mentioned before, N. glaciei is one of example microalgae that has its own antifreeze proteins (AFPs) to protect themselves from freezing damage (Kang and Raymond, 2004).
3. Conclusions
Microalgae are rich with their potential applications, therefore various microalgae are attempted to be maintained. Currently cryopreservation methods for microalgae cannot be generalized (Rastoll et al., 2013). In the cryopreservation of microalgae, many things should be considered: microalgae types, cell concentration, culture age before preservation, freezing rates, CPAs used and CPAs concentrations.
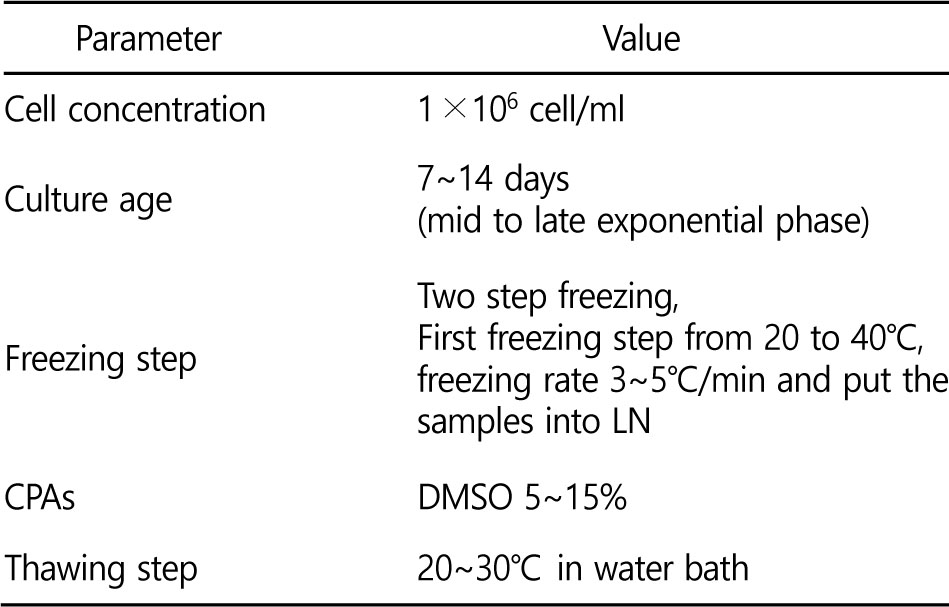
Among all kinds of CPAs, DMSO is the most universal CPA which is used successfully in the range of 5~15%. In the freezing step, two step freezing is more preferred than single step freezing since the latter can reduce the intracellular ice formation. The following parameters in the Table 3 may be used as starting parameters for microalgae cryopreservation.
Conflict of Interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
- References
-
4. Benson EE. 2008. Plant Cryopreservation: A Practical Guide (B. M. ReedEd.). Springer New York, New York, NY.
-
9. Day, JG, Benson EE, Harding K, Knowles B, Idowu M, Bremmer D, Santos L, Santos F, Friedl T, Lorenz M, Lukesova A, Elster J, Lukavsky J, Herdman M, Rippka R, Hall T. 2005. Cryopreservation and conservation of microalgae: the development of a pan-european scientific and biotechnological resource (the COBRA project). Cryo Letters 26: 231-238.
-
26. de Morais MG, Vaz B da S, de Morais EG, Costa JAV. 2015. Biologically Active Metabolites Synthesized by Microalgae. Bio- Med Research International 2015 1-15.
-
35. Sibinga CTS, Das PC, Greenwalt TJ (Eds.). 1986. Future Developments in Blood Banking. Springer US, Boston, MA.
-
36. Tanaka JY, Walsh JR, Diller KR, Brand JJ, Aggarwal SJ. 2001. Algae permeability to Me(2)SO from -3 to 23 degrees C. Cryobiology 42: 286-300.