JMLS 2024 June;9(1):41-46. 10.23005/ksmls.2024.9.1.41 Epub 2024 June 13
Copyright © 2024 by The Korean Society of Marine Life Science
Sex Change Scale and Pattern of Tegillarca granosa (Bivalvia : Arcidae)
Mi Ae Jeon; South Sea Fisheries Research Institute, National Institute of Fisheries Science, Yeosu 59780, Korea
Hyeon Jin Kim; Department of Aqualife Medicine, Chonnam National University, Yeosu 59626, Korea
So Ryung Shin; Department of Aqualife Medicine, Chonnam National University, Yeosu 59626, Korea
Jung Jun Park; Aquaculture Industry Research Division, East Sea Fisheries Research Institute, National Institute of Fisheries Science, Gangneung 25435, Korea
Hyun Park; Division of Biotechnology, College of Life Sciences and Biotechnology, Korea University, Seoul 02841, Korea
Jung Sick Lee; Department of Aqualife Medicine, Chonnam National University, Yeosu 59626, Korea
- Abstract
This study aimed to reconfirm the sex change scale and pattern of Tegillarca granosa. Although the sex ratio (female : male, female proportion) of T. granosa was 1:2.32 (30.2%) at the initial stage (2011) of the study, it was 1:0.94 (51.5%) after one year (2012) in the same population. The increase of the female proportion was greater in the 2+ year class (23.0%) when compared to the 1+ year class (19.2%). Overall, sex change ratio of 37.6% was observed in this population of T. granosa. The sex change ratio of the 2+ year class (39.3%) was higher than that of the 1+ year class (35.3%). And sex change ratio in the males (42.2%) was higher than that in the females (26.9%). The female proportion was the opposite of the result from 2006~2007, and one of the causes was presumed to be the difference in cumulative water temperature during the gonadal inactive stage (winter).
Keywords: Tegillarca granosa Sex ratio Sex change
Correspondence to: Jung Sick Lee; Department of Aqualife Medicine, Chonnam National University, Yeosu 59626, Korea
- Received
- 2 April 2024;
- Revised
- 5 April 2024;
- Accepted
- 7 May 2024.
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Language: Korean/English,
Full Text:

Introduction
The hermaphroditism is divided into simultaneous, and sequen- tial. Simultaneous hermaphroditism is the simultaneous release of eggs and sperm by one organism during the same season. Sequential hermaphrodites are either male or female for one or several annual cycles (Heller, 1993; Gosling, 2004; Collin, 2013). Sequential hermaphroditism has been reported in Patinopecten yessoensis (Osanai, 1975; Ventilla, 1982), Mercenaria mercenaria (Sastry, 1979; Menzel, 1989), Crassostrea rivularis, C. madrasensis, C. gigas, C. virginica, Saccostrea glomerata, S. cucculata, and Ostrea edulis (Mackie, 1984; Asif, 1979; Sastry, 1979; Guo et al., 1998; Park et al., 2012), Idas washingtonia (Tyler et al., 2009), Pinctada margaritifera (Chávez-Villalba et al., 2011) and Ruditpes philippinarum (Lee et al., 2013).
Lee et al. (2014) have presented direct evidence of sex changes in T. granosa and the fact that T. granosa is a sequential her- maphroditic bivalve undergoing sex change. However, definitive conclusion on whether the scale and pattern of sex change of T.granosa is always consistent could not be made. Therefore, the objective of this study was to reconfirm sex change in T. granosa and consider the scale and pattern of sex change compared to the results of Lee et al. (2014).
Tegillarca granosa is one of the important species for bivalve aquaculture in Korea, and its production is continuously declining from 5114 tons in 2010 to 1248 tons in 2015 and 80 tons in 2022 (KOSIS, 2023).
Materials and Methods
1. Specimens
Tegillarca granosa was sampled from a muddy intertidal region of Jangsu Bay on the southern coast of Korea (N34°65'04", E127°57'93"), the same research location of the study by Lee et al. (2014). The total number of T. granosa used for sex change identification was 777 with a shell length (SL) between 25.1~35.0 (30.9 ± 2.13) mm. The study was conducted by dividing specimens into two groups: a 1+ year class (14 months, SL: 28.6 ± 1.02 mm, n = 302) and a 2+ year class (26 months, SL: 32.2 ± 1.36 mm, n = 475).
2. Environmental conditions
Data from the Korea Hydrographic and Oceanographic Admin- istration (KHOA, 2012) were used for the water temperature and salinity profile in this study.
3. Sex change identification
Experiment for confirming sex change was conducted at the sampling location of T. granosa and the duration of rearing in the wild was approximately one year, from June 2011 to June 2012. Sex identification, tagging and confirmation of sex change were carried out in accordance with the methods of Lee et al. (2014).
4. Sex ratio
The sex ratio and female frequency were computed with the following equation.
Sex ratio = Female (n) : Male (n)
Female proportion (%) = [Female / Female + Male] × 100
5. Histological analysis
In addition to microscopic analysis, histological techniques were also used to confirm the sex of each specimen. The study organisms were dissected, and their visceral mass, which included the gonad, was fixed in aqueous Bouin's solution for 18 h and rinsed in running water for 24 h and then dehydrated through a graded ethanol series (70~100%, Duksan, Korea). The preparations were then embedded in paraplast (McCormick, U.S.A.). Embedded tissues were sectioned at 4~6 μm thickness using a microtome (RM2235, Leica, Germany). Samples were stained with Mayer's hematoxylin-0.5% eosin (H-E) stain (Sigma-Aldrich, Saint Louis, United States).
6. Statistical analysis
Statistical analyses were performed using SPSS 18.0 (SPSS Inc., U.S.A.). Sex change data were assessed by t-test. Significance was established at p<0.05.
Results
1. Environmental conditions
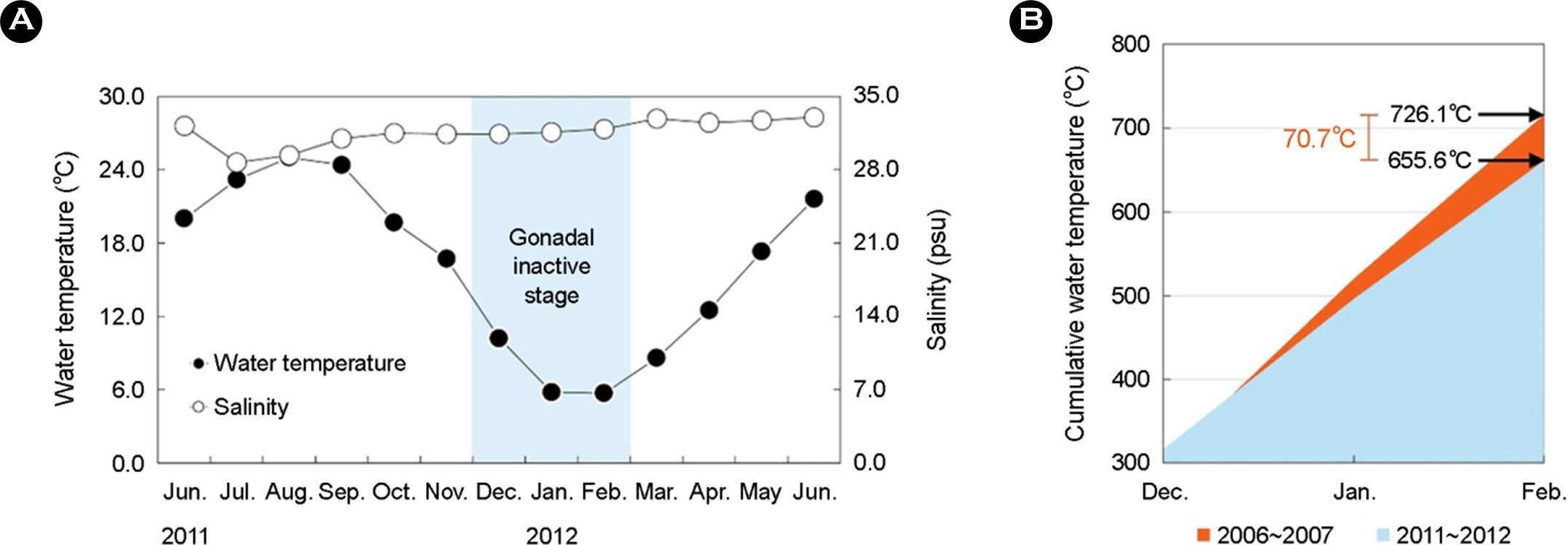
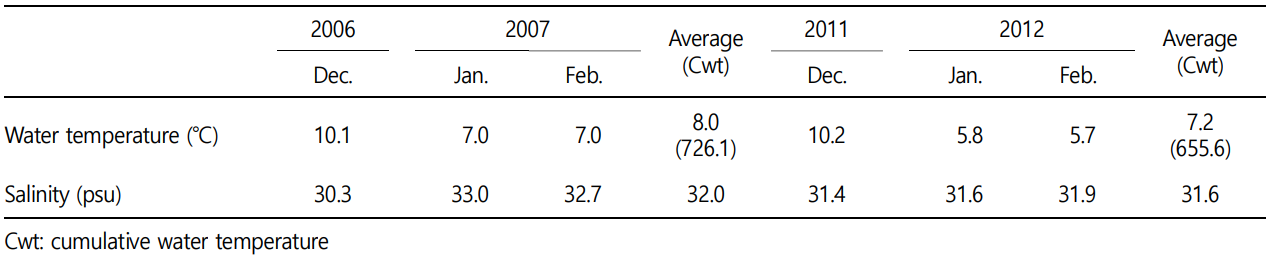
The average water temperature at the rearing location of ex-perimental organisms was 16.2℃ (5.7~24.4℃), similar to historical records. The average salinity was 31.6 psu (28.7~32.7 psu). The average and cumulative water temperature during the period of December to February, the gonadal inactive stage, was 7.2℃ (5.7~10.2℃) and 655.6℃, respectively. The average salinity during that period was 31.6 psu (31.4~31.9 psu) (Fig. 1A, Table 1).
2. Recovery rate and growth
As the result of having cultured T. granosa in the wild for approximately one year from June 2011 to June 2012, the overall recovery rate was 55.5% (n = 431/777). The recovery rate of the 1+ year class was 61.9% (n = 187/302) with SL increasing from 28.6 (± 1.02) mm to 31.6 (± 1.28) mm. In the 2+ year class, the recovery rate was 97.3% (n = 322/331) with SL increased from 32.2 (± 1.36) mm to 35.0 (± 1.73) mm.
3. Change of sex ratio (female : male)
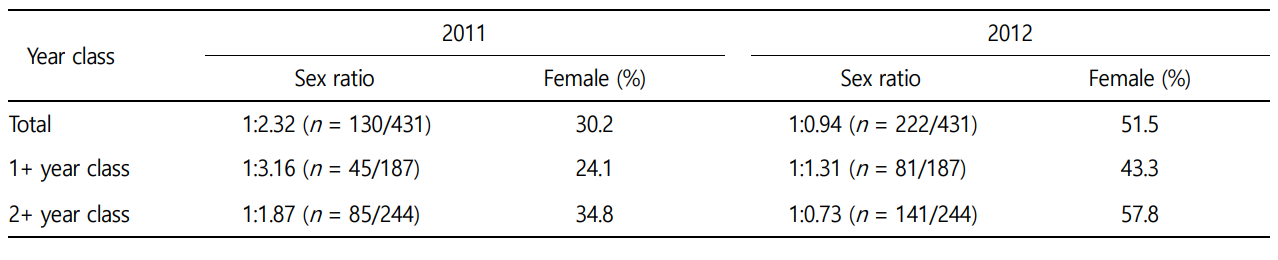
Although the sex ratio (female proportion) of T. granosa was 1:2.32 (30.2%) at the initial stage (2011) of the study, it was 1:0.94 (51.5%) after one year (2012) in the same population. When the analysis was based in "year classes", the female proportion in the 1+ year class at the initial stage of the study was 24.1% and in- creased to 43.3% after one year in the same population. Although the female proportion in the 2+ year class in 2011 was 34.8%, it also increased to 57.8% in 2012. However, the increase in the female proportion was significantly greater in the 2+ year class (23.0%) in comparison to that of the 1+ year class (19.2%) (Table 2).
4. Sex change ratio
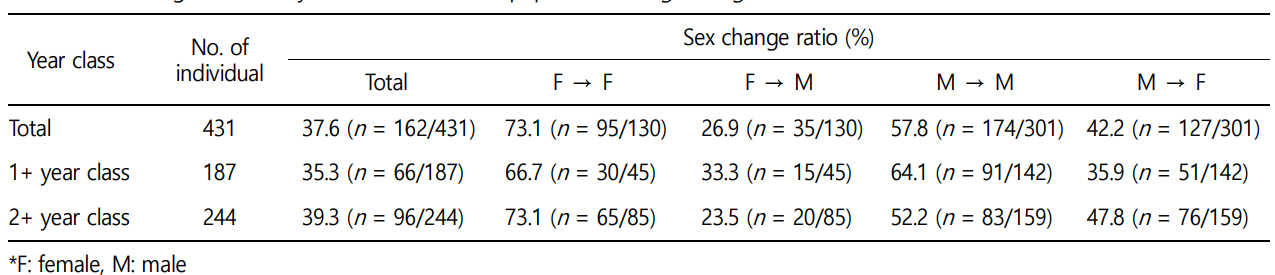
For the T. granosa population cultured in the wild for approxi- mately one year, the overall sex change ratio was 37.6%. Of these, the sex change ratio from females to males was 26.9% while the change from males to females was 42.2%. When the analysis was based on "year classes" the sex change ratio of the 1+ year class was 35.3%. Of these, the sex change ratio from females to males was 33.3% while that from males to females was 35.9%. The sex change ratio of the 2+ year class was 39.3%. Of these the sex change ratio from females to males was 23.5% and that from males to females was 47.8% (Table 3).
Discussion
The sequential hermaphroditism in bivalves signifies sex change (Heller, 1993; Gosling, 2004). The sex determination and sex change of bivalves are influenced by genetic factors and environmental factors such as water temperature and food (Zouros et al., 1992; Guo et al., 1998; Yusa, 2007; Stenyakina et al., 2010; Yusa et al., 2013). However, reports on the scale and pattern of the sex change of bivalves as well as the environmental factors involved in the sex change are limited.
Although sex change has been reported for other bivalves, such as scallop Patinopecten yessoensis (Osanai, 1975; Ventilla, 1982), sex change studies have traditionally been conducted on oysters. Historically, the literature points to the higher proportion of males at the early stage, with increasing proportion of females arising through sex change at older stages of oysters (Orton, 1933; Galtsoff, 1964; Thompson et al., 1996; Guo et al., 1998). Ostrea edulis has 10~16% male to female sex change in the first year with approximately 50% male to female sex change in the second year (Orton, 1933). They initially mature as males, and undergo sex change into females after the first discharge of sperm, while sex change is repeated throughout their life cycle (Walne, 1974). O. lurida undergoes rhythmical sex change in which the repetition of sex change between female and male phase occurs regularly, fol- lowing a preliminary male phase (Coe, 1934). Crassostrea virginica is a protandric species with an increase in the proportion of females through sex change as it ages (Thompson et al., 1996). Guo et al. (1998) found that the female proportion of C. gigas was 37%, 55%, and 75% in one-year, two-year, and three-year old oysters, respectively. This was assumed to be as a result of sex change from male to female. However, Park et al. (2012) con- firmed the sex change from male to female and then to male of C. gigas in the southern coast of Korea in accordance with their ages. Such results suggest the possibility that the patterns of sex change can differ depending on the habitat environment even for those of the same species.
In Veneridae, Mercenaria mercenaria becomes sexually mature at less than one year old, developing first as males but changing to an equal sex ratio in the second year (Menzel, 1989). For Ruditapes philippinarum, the sex ratio of the males is higher at first. The ratio of females increases with the increase of their age. Although its overall sex change ratio is approximately 19%, the sex change ratio from female to male is approximately 12.9% while that from male to female is approximately 25.9% (Lee et al., 2013).
For Tegillarca granosa, the scale of sex change during 2006~ 2007 was reported to be 15.1% (Lee et al., 2014). In this study, the overall scale of sex change in T. granosa was 37.6% during 2011~2012, which was approximately 2.5 times higher than that reported by Lee et al. (2014). In addition, the difference between the sex change ratio from females to males and that from males to females was 15.3% during the period of 2011~2012, which was similar to the finding of 15.0% during 2006~2007 (Lee et al., 2014). The sex change ratio of female to male : male to female was 1:3.42 during 2006~2007 reported by Lee et al. (2014). It was 1: 1.57 during 2011~2012 in this study.
It is impossible to confirm germ cells during the inactive stage of the sequential hermaphroditic bivalves including C. virginica (Thompson et al., 1996), R. philippinarum (Lee et al., 2013), and T. granosa (Lee et al., 2014). Moreover, they do not display histo- logical evidence of progressive sex change during the repro- ductive cycle and undergo the sex change as they go through the gonadal inactive stage. Therefore, environmental factors of their habitat during this stage might have effect on the scale and pattern of their sex change.
Water temperature imparts important effect on the items related to the reproduction including the sex determination, embryonic development, sex ratio, and sexual maturation of the molluscs (Mackie, 1984; Chávez-Villalba et al., 2003; Yusa, 2007). Such effect of water temperature on reproduction is continuous for prolonged period of time rather than temporary, thereby necessitating the employment of integral water temperature.
This study was executed by setting the venue and duration of the research that were the same as those of the research by Lee et al. (2014). And comparatively analyzed the data on of the water temperature and salinity, which are the representative factors of a marine environment closely associated with the reproduction of marine organisms between 2006~2007 and 2011~2012. As the results, although there was a difference of 0.8℃ in the average water temperature during the winter, the inactive stage, between 2006~2007 (8.07℃) and 2011~2012 (7.27℃), the corresponding average difference in the salinity was quite minor at 0.4 psu. When this difference in the water temperature during the inactive stage is computed as the cumulative water temperature, the difference in the water temperature for 2011~2012 (655.6℃) is 70.5℃ lower than that for 2006~2007 (726.1℃). Therefore, the difference in the water temperature during the inactive stage between 2006~2007 and 2011~2012 might be the cause of differences in the scale and patterns of the sex changes in T. granosa (Table 1, Fig. 1B).
In this study, it was possible to make considerations for water temperature and salinity on the based on existing data. However, other factors affecting the sex change scale of T. granosa cannot be completely ruled out. Therefore, regarding the effect of water temperature on their sex change, it is necessary to confirm the change in sex ratio by changing the rearing water temperature during the gonadal inactive stage.
- References
-
1. Asif M. 1979. Hermaphroditism and sex reversal in the four com- mon oviparous species of oysters from the coast of Karachi. Hydrobiologia 66: 49-55.
-
2. Chávez-Villalba J, Cochard JC, Le Pennec M, Barret J, Enriquez-Diaz M, Caceres-Martinez C. 2003. Effects of temperature and feeding regimes on gametogenesis and larval production in the oyster Crassostrea gigas. J Shellfish Res 22: 721-731.
-
3. Chávez-Villalba J, Soyez C, Huvet A, Gueguen Y, Lo C, Le Moullac G. 2011. Determination of gender in the pearl oyster Pinctada margaritifera. J Shellfish Res 30: 231-240.
-
-
5. Collin R. 2013. Phylogenetic patterns and phenotypic plasticity of molluscan sexual systems. Integr Comp Biol 53: 723-735.
-
6. Galtsoff PS. 1964. The American oyster Crassostrea virginica Gmelin. vol. 64. Government printing office, Washington, pp 1-480.
-
7. Gosling E. 2004. Bivalve Molluscs: Biology, Ecology and Culture. Blackwell Science, Oxford, pp 1-443.
-
8. Guo X, Hedgecock D, Hershberger WK, Cooper K, Allen Jr SK. 1998. Genetic determinants of protandric sex in the Pacific oyster, Crassostrea gigas Thunberg. Evolution 52: 394-402.
-
-
10. KHOA (Korea Hydrographic and Oceanographic Agency). 2012. Real time coastal data; Yeosu. [Online] URL http://www.khoa. go.kr/koofs/kor/observation/obs_past_search_statistic.asp.
-
11. KOSIS (Korean Statistical Information Service). Available online: https://kosis.kr/index/index.do (accessed on 20 March 2023).
-
12. Lee JS, Park JJ, Shin YK, Kim H, Jeon MA. 2014. Sex change and sequential hermaphroditism in Tegillarca granosa (Bivalvia: Arcidae). Invertebr Reprod Dev 58: 314-318.
-
13. Lee JS, Park JS, Shin YK, Lee YG, Park JJ. 2013. Sequential hermaphroditism in Manila clam Ruditapes philippinarum (Bivalvia : Veneridae). Invertebr Reprod Dev 57: 185-188.
-
14. Mackie GL. 1984. Bivalves. Tompa AS, Verndonk NH, Biggelaar JAM (eds.), The Mollusca, Reproduction. vol. 7. Academic Press Inc., New York, pp 351-418.
-
15. Menzel W. 1989. The Biology, fishery and culture of Quahog clam, Mercenaria. Manzi JJ, Castagna M (eds.), Clam Culture in North America. Elsevier Science Publishers, Amsterdam, pp 201-242.
-
16. Orton JH. 1933. Observations and experiments on sex-change in the European oyster (O. edulis). Part III. On the fate of un- spawned ova. Part IV. On the change from male to female. J Mar Biol Assoc 6: 1-54.
-
17. Osanai K. 1975. Seasonal gonad development and sex alteration in the scallop Patinopecten yessoensis. Bull Mar Biol Stn Asamushi 15: 81-88.
-
18. Park JJ, Kim H, Kang SW, An CM, Lee SH, Gye MC, Lee JS. 2012. Sex ratio and sex reversal in two-year-old class of oyster, Crassostrea gigas (Bivalvia: Ostreidae). Dev Reprod 16: 385-388.
-
19. Sastry AN. 1979. Pelecypoda (excluding Ostreidae). Giese AG, Pearse PJ (eds.), Reproduction of Marine Invertebrates. vol. 5. Academic Press Inc., New York, pp 113-292.
-
20. Stenyakina A, Walters LJ, Hoffman EA, Calestani C. 2010. Food availability and sex reversal in Mytella charruana, an intro- duced bivalve in the southeastern United States. Mol Reprod Dev 77: 222-230.
-
21. Thompson RJ, Newell RIE, Kennedy VS, Mann R. 1996. Repro- ductive process and early development. Kennedy VS, Newell RIE, Eble AF (eds.), The Eastern Oyster Crassostrea virginica. Maryland Sea Grant College, Maryland, pp 335-370.
-
22. Tyler PA, Marsh L, Baco-Taylor A, Smith CR. 2009. Protandric hermaphroditism in the shale-fall bivalve mollusc Idas wash- ingtonia. Deep-Sea Res II: Top Stud Oceanogr 256: 1689-1699.
-
-
24. Walne PR. 1974. Culture of Bivalve Molluscs: 50 years' Experience at Conwy. Fishing News Books Ltd., Surrey, pp 1-189.
-
25. Yusa Y. 2007. Causes of variation in sex ratio and modes of sex determination in the Mollusca-an overview. Am Malacol Bull 23: 89-98.
-
26. Yusa Y, Breton SR, Hoeh W. 2013. Population genetics of sex determination in Mytilus mussels: reanalyses and a model. J Hered 104: 380-385.
-
27. Zouros E, Freeman KR, Ball AO, Pogson GH. 1992. Direct evidence for extensive paternal mitochondrial DNA inheritance in the marine mussel Mytilus. Nature 359: 412-414.