JMLS 2023 December;8(2):190-195. 10.23005/ksmls.2023.8.2.190 Epub 2023 December 17
Copyright © 2023 by The Korean Society of Marine Life Science
Acute Exposure to Karenia mikimotoi Induces Oxidative Stress and Reduces Immune Parameters in the Marine Medaka Oryzias javanicus
Seong Duk Do1, ; Department of Marine Science, College of Natural Sciences, Incheon National University, Incheon 22012, Korea
Yun Kyung Shin2, ; National Institute of Fisheries Science, Busan 46083, Korea
Jae-Sung Rhee1,3,4*; Department of Marine Science, College of Natural Sciences, Incheon National University, Incheon 22012, Korea; Research Institute of Basic Sciences, Incheon National University, Incheon 22012, Korea; Yellow Sea Research Institute, Incheon 22012, Korea
- Abstract
In this research, the marine medaka Oryzias javanicus underwent a 96 h exposure to two concentrations of the red tide dinoflagellate Karenia mikimotoi (1,000 and 5,000 cells mL-1), and the temporal variations in biochemical responses related to antioxidant and immunity parameters were assessed in the liver tissue. The study revealed a significant increase in ichthyotoxicity with elevated cell concentrations of K. mikimotoi, especially evident at 96 h in marine medaka exposed to 5,000 cells mL-1. At 1,000 cells mL-1 of K. mikimotoi, the opercular respiratory rate showed a significant increase, whereas exposure to 5,000 cells mL-1 resulted in a lowered rate. The intracellular malondialdehyde content was significantly elevated in response to both cell concentrations at 96 h. Regarding glutathione content, levels were significantly increased by exposure to both cell concentrations. Catalase and superoxide dismutase enzymatic activities experienced an increase at 1,000 cells mL-1 of K. mikimotoi, while their activities were reduced at 5,000 cells mL-1 at 96 h. The analysis of two immunity parameters, alternative complement pathway and lysozyme, demonstrated significantly reduced activities in the liver tissue exposed to 5,000 cells mL-1 of K. mikimotoi. These findings aim to enhance the understanding of K. mikimotoi toxicity in marine fish by offering insights into biochemical responses associated with harmful algal blooms.
Keywords: Karenia mikimotoi Red tide Marine medaka Oxidative stress Iimmunity
Correspondence to: Jae-Sung Rhee; Department of Marine Science, College of Natural Sciences, Incheon National University, Incheon 22012, Korea
- Received
- 20 November 2023;
- Revised
- 24 November 2023;
- Accepted
- 28 November 2023.
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Language: Korean/English,
Full Text:

Harmful algal blooms (HABs) result from the excessive prolif- eration of various dinoflagellates, diatoms, raphidophytes, or other taxonomic groups in aquatic ecosystems, when environmental conditions favoring their blooms are established (Landsberg, 2002). These blooms, particularly associated with dinoflagellates, can inflict direct damage or release toxins that lead to mass mortalities in fish and shellfish, impacting aquaculture and fisheries (Hallegraeff, 2003). The global significance of the detrimental effects of HABs on economically vital aquaculture and fisheries has been under- scored (Whyte et al., 2001; Kudela and Gobler, 2012). Dinofla- gellates, diverse and abundant marine planktons, play crucial roles as food sources in aquatic trophic levels (Shumway, 1990). HABs induced by dinoflagellates, often referred to as red tides, can pro- duce ichthyotoxic compounds even at low cell densities, leading to harmful effects through bioaccumulation in various trophic levels, including marine mammals (Landsberg, 2002; Hallegraeff, 2003; Mindy et al., 2010). Fish exposed to dinoflagellates exhibit various physical and physiological damages, such as direct physical contact with gill tissues, epithelial hyperplasia, gill clogging, anoxia, convulsions, swelling and necrosis of the lamellar epithelium, osmoregulatory disruption, and excess mucus secretion (Kim et al., 1999; Chen and Chou, 2001; Cembella et al., 2002; Landsberg, 2002; Band-Schmidt et al., 2003; Gobler et al., 2008; Shin et al., 2019; Haque et al., 2023).
Karenia mikimotoi is a prevalent species of red tide dinofla- gellate known for inducing mass mortality among marine fauna in the coastal waters of the Republic of Korea (Shin et al., 2023). In this study, we aimed to analyze the acute effects of K. mikimotoi on the liver tissue of the marine medaka, Oryzias javanicus. While potential effects of K. mikimotoi have been reported in marine fish, such as red seabream (Shin et al., 2023), the underlying molecular mechanisms remain poorly understood. To assess potential haz- ardous effects, we conducted biochemical assays targeting the antioxidant defense system and innate immunity, using concen- trations of 1,000 and 5,000 cells mL-1 of K. mikimotoi. Intracellular contents of malondialdehyde (MDA) and glutathione (GSH) were monitored with analysis of enzymatic activities of catalase (CAT) and superoxide dismutase (SOD) to provide evidence of K. mikimotoi-mediated fluctuations in redox homeostasis. In addition, GSH-mediated antioxidant responses, along with two immune parameters-alternative complement pathway (ACH50) and lysozyme -were analyzed in the liver tissue following exposure to 1,000 and 5,000 mL-1 of K. mikimotoi.
Entire animal handling and experimental procedures adhered to the ethical standards and were approved by the Animal Experi- mental Ethics Committee of Incheon National University (Incheon, South Korea). The marine medaka, Oryzias javanicus, utilized in this study were housed in artificial seawater (TetraMarine Salt Pro, Cincinnati, OH, USA) with a practical salinity of 31 ± 0.7 units and dissolved oxygen levels of 5.98 ± 0.69 mg O2 L-1, maintained at a temperature of 20 ± 1℃ under a 14/10 h light/darkness cycle. The fish were provided with frozen mosquito larvae and an artificial diet twice daily until reaching satiation.
A laboratory culture of K. mikimotoi was conducted following the methods outlined in a previous study (Shin et al., 2023). The dinoflagellate was cultivated at 20℃ in 0.001 μM f/2 medium, dissolved in filtered seawater, under continuous light conditions at 100 μmol photons/m2/s. The cell count of K. mikimotoi was determined using a Leica DMLA microscope (Leica Microsystems, Wetzlar, Germany) equipped with UV epifluorescence and an AxioCam HRc camera (Zeiss, Göttingen, Germany) employing a Sedgwick-Rafter counting chamber (VWR, Langenfeld, Germany). For exposure experiments, K. mikimotoi was harvested during the exponential and early stationary phases to achieve final concen- trations equivalent to approximately 10,000 K. mikimotoi cells mL-1 before treatment.
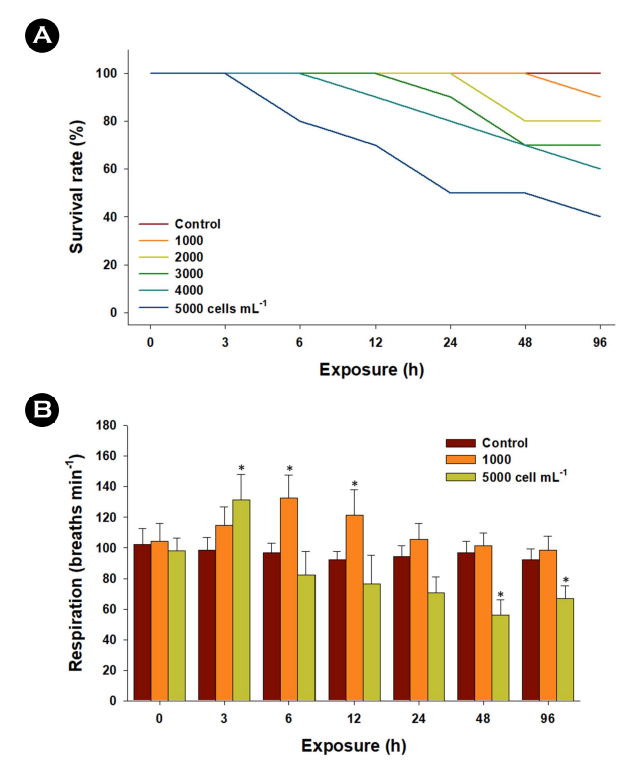
To assess sublethal effects, individual exposure experiments were conducted using 30 marine medaka. The fish were subjected to varying concentrations, including a control group and concen- trations of 1,000, 2,000, 3,000, 4,000, and 5,000 cells mL-1 of K. mikimotoi for 96 h. Throughout the exposure period, the fish were not provided with any food. No mortality was observed in the control group during the 96 h experimental period. Survival rates were recorded at intervals of 0, 3, 6, 12, 24, 48, and 96 h for both the control and the exposed marine medaka groups. Opercular respiratory rates were measured in marine medaka exposed to concentrations of 1,000 and 5,000 cells mL-1. This measurement was conducted in a transparent chamber where ten marine medaka from each concentration group were placed. The respiratory beats of each marine medaka were recorded over a 5-min period.
For the assessment of biochemical responses, sixty-three marine medaka were allocated to three groups corresponding to each concentration of K. mikimotoi. At intervals of 0, 3, 6, 12, 24, 48, and 96 h, three marine medaka from each concentration were collected. To obtain liver tissue samples, the individuals were anesthetized through immersion in a solution of tricaine methane- sulfonate (Sigma-Aldrich, Inc., St. Louis, MO, USA). Subsequently, each marine medaka was dissected to extract liver tissues. For the analysis, three liver samples from each subgroup were combined, and these pooled specimens were subjected to analysis in triplicate.
Biochemical responses were assessed following the method- ology outlined in our prior study (Do et al., 2022). In brief, liver tissues, combined and homogenized from each group, underwent homogenization in a cold buffer (20 mM Tris, 150 mM NaCl, 10 mM β-mercaptoethanol, 20 μM leupeptin, 2 μM aprotinin, and 100 μM benzamidine) and were then centrifuged for 30 minutes at 30,000 × g at 4℃. Following a 15 min heat denaturation of the supernatants at 75℃, thiobarbituric acid reactives (TBARs) were quantified at 535 nm using a Thermo Varioskan Flash spectro- photometer (Thermo Fisher Scientific, Tewksbury, MA, USA), with malonaldehyde bis (MDA; tetrametoxypropan, Sigma-Aldrich, Inc.) serving as a standard. The overall content of lipid peroxidation compounds was determined as nM of MDA per gram of liver tissue. Total soluble protein was gauged using the Bradford method. The intracellular GSH content in the combined liver tissues was measured employing a Glutathione Assay Kit (Catalog No. CS0260; Sigma-Aldrich, Inc.). Enzymatic activities of CAT and SOD were assessed through enzymatic methods using the SOD Assay Kit (Catalog No. 19160; Sigma-Aldrich Chemie, Switzerland) and the Catalase Assay Kit (Catalog No. CAT100; Sigma-Aldrich, Inc.), respectively. The ACH50 activity of K. mikimotoi-exposed marine medaka was analyzed using sheep red blood cells (SRBCs; 1.5 × 106 cells, National Institute of Toxicological Research, South Korea) following a previously established method (Nam et al., 2020). The lysozyme activity of K. mikimotoi-exposed marine medaka was measured using a turbidimetric assay in a 96-well plate (Ellis, 1990). All data were analyzed using the statistical software pack- age SPSS (ver. 17.0, SPSS Inc., Chicago, IL, USA) and expressed as mean ± standard deviation (S.D.).
Concentration-dependent mortality was evident with concen- trations ranging from 1,000 to 5,000 cells mL-1 of K. mikimotoi (Fig. 1A). This outcome implies a threshold for both cell concentration and exposure duration of K. mikimotoi concerning its ichthyo- toxicity in marine medaka. The opercular respiratory rates of marine medaka were significantly elevated at 3~12 h when exposed to 1,000 or 5,000 cells mL-1 of K. mikimotoi (p < 0.05). However, these rates were significantly reduced at 48 and 96 h in marine medaka exposed to 5,000 cells mL-1 (p < 0.05) (Fig. 1B). Con- sequently, the mortality observed in marine medaka exposed to 5,000 cells mL-1 is likely associated with significant damage to gill tissues. This damage may include alterations in gill epithelium structure, disruption of ionic homeostasis, and suffocation caused by the attachment of K. mikimotoi. The increased mortality is likely a result of the failure of gill function and respiration due to the direct ingestion and attachment/aggregation of K. mikimotoi. The detrimental effects on fish gill tissues are critical for mortality, as gills play a vital role in the absorption and transport of oxygen throughout the entire body.
Significant increases in MDA content were observed in liver tissues exposed to 1,000 cells mL-1 of K. mikimotoi at 96 h and 5,000 cells mL-1 at 24, 48, and 96 h (p < 0.05) (Fig. 2A). MDA is a byproduct of lipid peroxidation, and its measurement serves as a widely employed biomarker for assessing oxidative stress in fish (Lushchak, 2011). The elevated MDA content observed in marine medaka indicates that exposure to K. mikimotoi may induce the production of oxidative stressors, such as free radicals or pro-oxidant molecules, which can subsequently trigger lipid peroxidation.
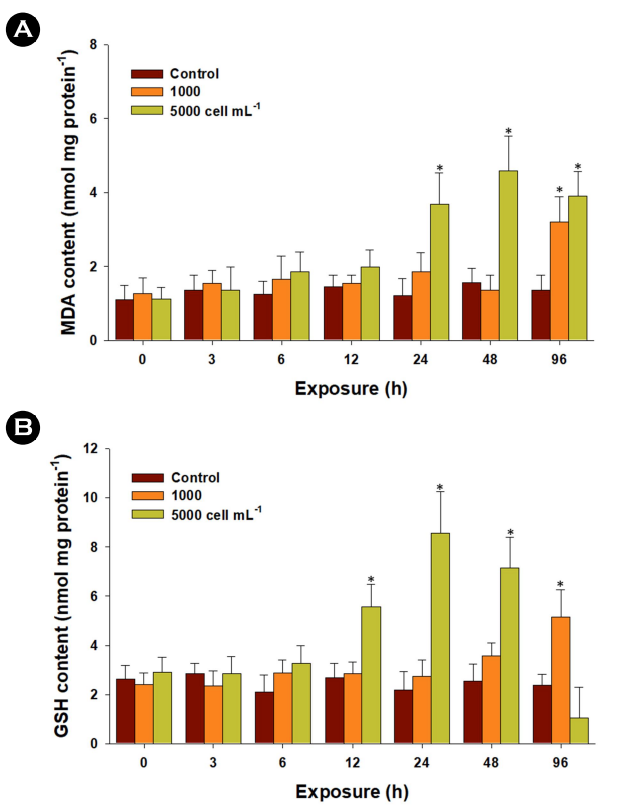
Regarding GSH content, a crucial non-enzymatic antioxidant that acts as a vital protector against cellular redox-cycling fluc- tuations induced by exogenous oxidative stressors (Lushchak, 2011), exposure to 1,000 cells mL-1 of K. mikimotoi resulted in significantly higher levels at 96 h (p < 0.05). In addition, the content significantly elevated at 12, 24, and 48 h when exposed to 5,000 cells mL-1 (p < 0.05) (Fig. 2B). The notable increase in GSH levels observed in marine medaka suggests the synthesis of new GSH, possibly needed to maintain the required levels for increased resistance capacity against oxidative stress as a free radical scavenger. The strongly modulated respiration rate could be con- sidered a factor in intracellular oxidative stress, as low oxygen-triggered lipid peroxidation and subsequent oxidative stress have been well-characterized in fish (Lushchak and Bagnyukova, 2006).
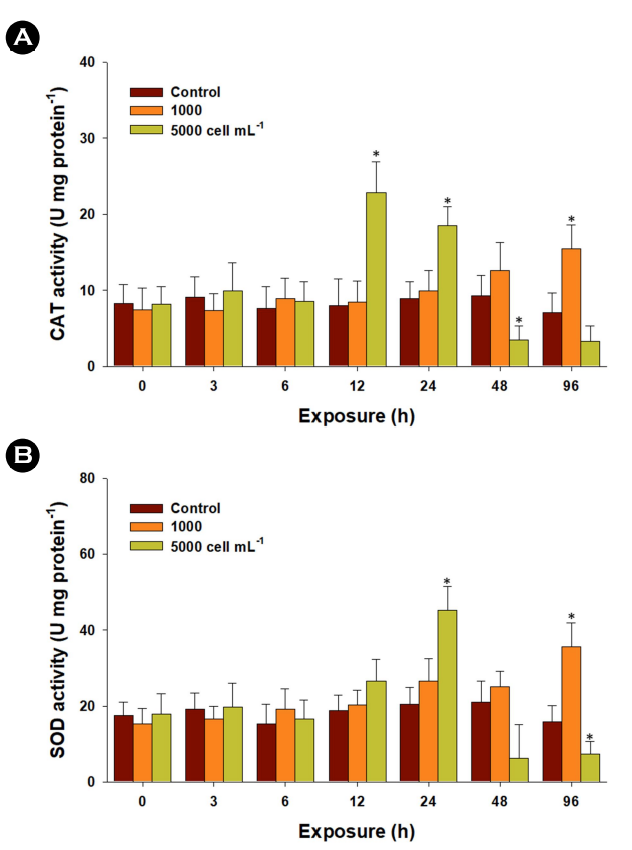
The two antioxidant parameters, CAT and SOD enzymes, con- stitute the primary line of defense against oxidative stress. In the liver tissue exposed to 1,000 cells mL-1 of K. mikimotoi, CAT activity exhibited a significant increase at 96 h (p < 0.05). The activity was also markedly elevated at 12 and 24 h but experienced a re- duction with exposure to 5,000 cells mL-1 at 48 and 96 h (Fig. 3A). Furthermore, upon exposure to 1,000 cells mL-1 of K. mikimotoi, there was a significantly higher activity of the SOD enzyme detected from 6 to 96 h (p < 0.05) (Fig. 3B). Additionally, a significantly higher level of SOD activity was measured in the liver tissue exposed to 5,000 cells mL-1 of K. mikimotoi at 24 h (p < 0.05). However, the activity of the SOD enzyme significantly decreased with exposure to 5,000 cells mL-1 of K. mikimotoi at 96 h (p < 0.05). Specifically, SOD transforms two superoxide radicals into hydrogen peroxide, and CAT breaks down hydrogen peroxide into water and molecular oxygen, thereby mitigating the adverse effects of free radicals (Lesser, 2006; Lushchak, 2011). The observed increase in enzymatic activities of CAT and SOD in marine medaka suggests a stress response and adaptive metab- olism to counteract oxidative stress. In the gill cell line RTgill-W1 exposed to the raphidophyte Chattonella marina, a significant increase in SOD enzyme activity was noted (Dorantes-Aranda et al., 2015). However, their concurrent reduction in CAT and SOD enzymes might be indicative of a failure in regulating oxidative homeostasis.
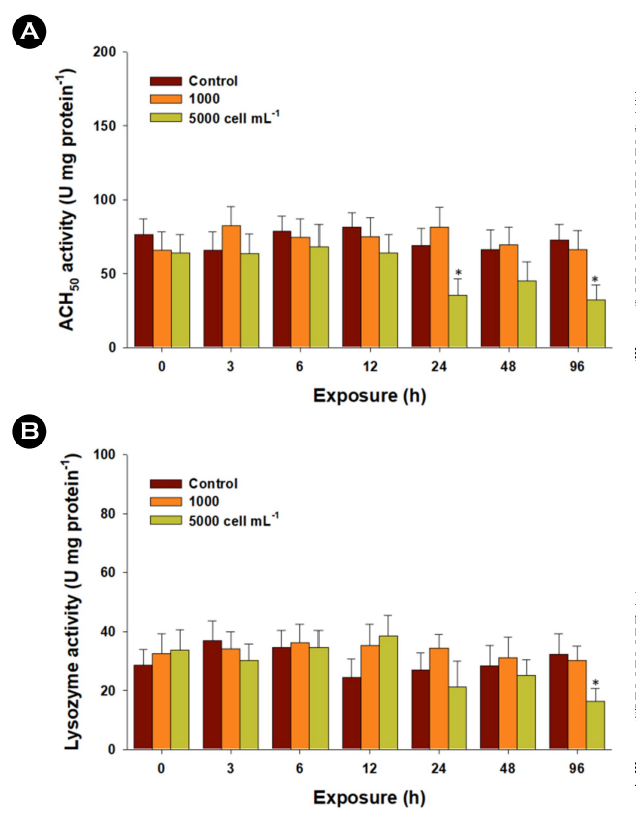
In general, fish experience immunological fluctuations in re- sponse to environmental stressors, although studies on HAB-triggered immune responses are limited. The immune parameters ACH50 and lysozyme play crucial roles in innate immunity, im- munosuppression, and homeostasis in fish (Boshra and Sunyer, 2006; Saurabh and Sahoo, 2008). The complement system is pivotal in innate immunity, offering a broad spectrum of immune recognition capabilities. A significant decrease in ACH50 activity was observed in liver tissues exposed to 5,000 cells mL-1 of K. mikimotoi at 24 and 96 h (p < 0.05) (Fig. 4A). Lysozyme activity measurement is a consistent method for monitoring the potential impact of exogenous stress on innate immunity. Similarly, exposure to 5,000 cells mL-1 of K. mikimotoi resulted in significantly lower levels at 96 h (p < 0.05) (Fig. 4B). The noteworthy decreases in enzymatic activities of ACH50 and lysozyme indicate K. mikimotoi-triggered immune suppression. As the liver is a crucial organ synthesizing complement components (Boshra and Sunyer, 2006), the significantly decreased ACH50 activity implies a potential dis- ruption in liver function. In fish, macrophages, neutrophils, and monocytes release lysozymes that directly lyse or degrade path- ogen cell walls (Magnadóttir, 2006). Previously, the dinoflagellate Alexandrium affine (6,000 and 7,000 cells mL-1) induced immuno- suppression by significantly decreasing lysozyme activity and total Ig levels in the gill and liver tissues of red seabream, Pagrus major (Haque et al., 2021). Therefore, the reduced lysozyme activity signifies significant immune suppression, potentially increasing the vulnerability of marine medaka to diseases and exogenous pathogens, allowing unchecked inflammation.
To mitigate the detrimental effects of HABs and subsequent economic losses, it is crucial to comprehend the precise ichthyo- toxicity mechanisms. In summary, all parameters of the antioxidant defense system and immunity analyzed in the liver tissue support the induction of oxidative stress and immunosuppression by K. mikimotoi in this species. Our results also imply that even environ- mental concentrations of HABs can inhibit respiration rates and may induce mass mortality in fish. Detecting early biological signals in aquatic animals in response to HABs will be useful in predicting the occurrence of HABs and establishing prevention plans in aquaculture and fisheries.
- References
-
1. Band-Schmidt CJ, Lechuga-Deveze CH, Kulis DM, Anderson DM. 2003. Culture studies of Alexandrium affine (Dinophyceae), a non-toxic cyst forming dinoflagellate from Bahía concepción, Gulf of California. Bot Mar 46: 44-54.
-
2. Boshra H, Li J, Sunyer JO. 2006. Recent advances on the com- plement system of teleost fish. Fish Shellfish Immunol 20: 239-262.
-
3. Cembella AD, Quilliam MA, Lewis NI, Bauder AG, Dell'Aversano C, Thomas K, Jellett J, Cusack RR. 2002. The toxigenic marine dinoflagellate Alexandrium tamarense as the probable cause of mortality of caged salmon in Nova Scotia. Harmful Algae 1: 313-325.
-
4. Chen CY, Chou HN. 2001. Ichthyotoxicity studies of milkfish Chanos chanos fingerlings exposed to a harmful dino- flagellate Alexandrium minutum. J Exp Mar Biol Ecol 262: 211-219.
-
5. Do SD, Shin YK, Rhee J-S. 2022. Effects of Cochlodinium poly- krikoides on oxidative status and immune parameters in the marine medaka Oryzias javanicus. J Mar Life Sci 7: 94-101.
-
6. Dorantes-Aranda JJ, Seger A, Mardones JI, Nichols PD, Hallegraeff GM. 2015. Progress in understanding algal bloom-mediated fish kills: The role of superoxide radicals, phycotoxins and fatty acids. PLoS One 10: e0133549.
-
7. Ellis AE. 1990. Lysozyme assays. In: Stolen JS, Fletcher TC, Anderson DP, Roberson BS, Van Muiswinkel WB. (Eds.), Techniques in Fish Immunology. SOS Publications, Fair Haven NJ, pp 101-103.
-
8. Gobler CJ, Berry DL, Anderson OR, Burson A, Koch F, Rodgers BS, Moore LK, Goleski JA, Allam B, Bowser P, Tang Y, Nuzzi R. 2008. Characterization, dynamics, and ecological impacts of harmful Cochlodinium polykrikoides blooms on eastern Long Island, NY, USA. Harmful Algae 7: 293-307.
-
9. Hallegraeff GM. 2003. Harmful algal blooms: a global overview, in Hallegraeff GM, Anderson DM, Cembella AD. (Eds.), Manual on harmful marine microalgae. UNESCO, Paris, France, pp 25-49.
-
10. Haque MN, Nam S-E, Shin YK, Rhee J-S. 2021. The dinoflagellate Alexandrium affine acutely induces significant modulations on innate immunity, hepatic function, and antioxidant de- fense system in the gill and liver tissues of red seabream. Aquat Toxicol 240: 105985.
-
11. Haque MN, Nam S-E, Lee M, Kim H-W, Gil H-W, Park HS, Rhee J-S. 2023. Chronic exposure to environmental concentrations of harmful algal bloom-forming dinoflagellates induces oxidative stress and reduces immune and hepatic functions in red seabream. Comp Biochem Physiol C 266: 109573.
-
12. Kim CS, Lee SG, Kim HG, Jung J. 1999. Reactive oxygen species as causative agents in the ichthyotoxicity of the red tide dinoflagellate Cochlodinium polykrikoides. J Plankton Res 21: 2105-2115.
-
13. Kudela R, Gobler C. 2012. Harmful dinoflagellate blooms caused by Cochlodinium sp.: global expansion and ecological strategies facilitating bloom formation. Harmful Algae 14: 71-86.
-
14. Landsberg JH. 2002. The effects of harmful algal blooms on aquatic organisms. Rev Fish Sci 10: 113-390.
-
15. Lesser MP. 2006. Oxidative stress in marine environments: bio- chemistry and physiological ecology. Annu Rev Physiol 68: 253-278.
-
16. Lushchak VI, Bagnyukova TV. 2006. Effects of different environ- mental oxygen levels on free radical processes in fish. Comp Biochem Physiol B 144: 283-289.
-
17. Lushchak VI. 2011. Environmentally induced oxidative stress in aquatic animals. Aquat Toxicol 101: 13-30.
-
-
19. Mindy LR, Steve LM, Ebrahim AJ, Anbiah R, Donald MA. 2010. The catastrophic 2008-2009 red tide in the Arabian Gulfregion, with observations on the identification and phylogeny of the fish killing dinoflagellate Cochlodinium polykrikoides. Harmful Algae 9: 163-172.
-
20. Nam S-E, Saravanan M, Rhee J-S. 2020. Benzo[a]pyrene con- strains embryo development via oxidative stress induction and modulates the transcriptional responses of molecular biomarkers in the marine medaka Oryzias javanicus. J Environ Sci Health A 55: 1050-1058.
-
21. Saurabh S, Sahoo PK. 2008. Lysozyme: an important defence molecule of fish innate immune system. Aquac Res 39: 223-239.
-
22. Shin YK, Nam S-E, Kim WJ, Seo DY, Kim Y-J, Rhee J-S. 2019. Red tide dinoflagellate Cochlodinium polykrikoides induces sig- nificant oxidative stress and DNA damage in the gill tissue of the red seabream Pagrus major. Harmful Algae 86: 37-45.
-
23. Shin YK, Seo DY, Eom H-J, Park M, Lee M, Choi Y-E, Han Y-S, Rhee J-S, Kim Y-J. 2023. Oxidative stress and DNA damage in Pagrus major by the dinoflagellate Karenia mikimotoi. Toxins 15: 620.
-
24. Shumway SE. 1990. A review of the effects of HABs on shellfish and aquaculture. J World Aquacult Soc 21: 65-104.
-
25. Whyte JNC, Haigh N, Ginther NG, Keddy LJ. 2001. First record of blooms of Cochlodinium sp. (Gymnodiniales, Dinophyceae) causing mortality to aquacultured salmon on the west coast of Canada. Phycologia 40: 298-304.