JMLS 2016 April;1(1):41-49. Epub 2016 April 30
Copyright © 2016 by The Korean Society of Marine Life Science
Expression of Maturation-Related Genes and Leptin during Sexual Maturation in the Female Goldfish: Effects of Exogenous Kisspeptin
Na Na Kim; Division of Marine BioScience, Korea Maritime and Ocean University, Busan 49112, Korea
Young Jae Choi; Division of Marine BioScience, Korea Maritime and Ocean University, Busan 49112, Korea
Sung-Yong Oh; Ecosystem and Biological Research Center, Korea Institute Ocean Science & Technology, Ansan 15627, Korea
Cheol Young Choi; Division of Marine BioScience, Korea Maritime and Ocean University, Busan 49112, Korea
- Abstract
Kisspeptin (Kiss) and its cognate receptor, kisspeptin receptor (KissR; G protein coupled receptor 54, GPR54), have recently been recognized as potent regulators of reproduction in teleosts. Additionally, leptin plays an important role in energy homeostasis and reproductive function in teleosts. The purpose of this study was to examine differences in the concentration of the hormones of the Kiss/KissR system and leptin and the expression of their underlying genes, all of which are involved in the sexual maturation of female goldfish, Carassius auratus, following treatment with Kiss. The expression levels of KissR increased after the Kiss injection. Furthermore, the peptide hormone leptin also increased after the injection (in vivo and in vitro). Additionally, the expression of GnRH and GTHs (GTHα, FSHβ, and LHβ) increased in the brain and pituitary (in vitro and in vitro). These results support the hypothesis that Kiss plays important roles in the direct regulation of the hypothalamus-pituitary-gonad axis and leptin in goldfish. Therefore, we suggest that Kiss system gene expression is correlated with energy balance and reproduction.
Keywords: Goldfish Hypothalamus-pituitary-gonad axis Kiss/KissR Leptin Sexual maturation
Correspondence to: Cheol Young Choi; Division of Marine BioScience, Korea Maritime and Ocean University, Busan 49112, Korea, E-mail : choic@kmou.ac.kr
- Received
- 1 April 2016;
- Revised
- 14 April 2016;
- Accepted
- 22 May 2016.
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0/) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Introduction
Recently, kisspeptin (Kiss), a neuropeptide that regulates sexual differentiation and spawning time in vertebrates (Pasquier et al.,2011; Chang et al., 2012; Kim et al., 2014), and its receptor, KissR (GPR54, G-protein coupled receptor 54), have been shown to play major roles in the central regulation of the hypothalamus-pituitarygonad (HPG) axis (Roa et al., 2011; Chang et al., 2012). Kiss, a member of the Arg-Phe (RF)-amide peptide family, is located in the pre-optic area of the hypothalamus, and it controls sexual maturation and regulation factors (Roa et al., 2011). In addition, the Kiss-GPR54 complex regulates gonadotropin-releasing hormone (GnRH) expression through nerve activation, which allows it to play a role as a sexual maturation and regulation factor (Colledge, 2009).
There are two types of Kiss isoforms (Kiss1 and Kiss2) along with their receptors (KissR-1 and KissR-2, respectively) in teleosts (Um et al., 2010). Kiss1 is an important neuroendocrine factor that regulates sexual maturation and plays a role in initiating sexualmaturation of the GnRH neurons in the hypothalamus (Colledge, 2009; Roa et al., 2011). Histological study has shown that Kiss1 and KissR are in the same location as the GnRH neurons, whichsupports the hypothesis that Kiss-KissR interacts with GnRH (Irwig et al., 2004; Messager et al., 2005). In addition, mRNA expression of follicle-stimulating hormone β (FSHβ) and luteinizing hormone β (LHβ) in the pituitary is regulated by Kiss2 in zebrafish, Danio rerio (Kitahashi et al., 2009), and this gene plays an important role in regulating reproduction and stimulating LH secretion before reproduction and ovulation (Chang et al., 2012).
Leptin, the appetite-suppressing hormone, is known to affect metabolism and feeding activity, as well as reproduction (Copeland et al., 2011). In particular, the amount of leptin expressed increases during sexual maturation (Blüher and Mantzoros, 2007). Approximately 40% of leptin receptors are distributed around Kiss neurons. As such, it is expected that leptin affects the propagation and control of early maturation and the HPG axis in connection with Kiss (Smith et al., 2006). Smith et al. (2006) reported that when mice that were leptin-deficient (owing to a genetic defect) were given an injection of 2 μg g-1 leptin, Kiss expression increased, suggesting that there may be a relationship between leptin and Kiss.
A previous study, focusing on the role of leptin in sexual maturation and reproduction in fish, reported that the human leptin protein, acting at the level of the pituitary, directly stimulated FSH and LH release in rainbow trout, Oncorhynchus mykiss (Weil et al.,2003). However, information on the correlation of Kiss and leptin effects and the mechanism of sexual maturation is still lacking. Clarkson and Herbison (2006) stated a need for studying the mech anisms of Kiss/KissR and leptin with regard to regulating aspects related to neuroendocrinology, including feedback control of gonadotropin secretion and sexual dimorphism in vertebrates.
In this study, we examined the relationship between Kiss/KissR (GPR54) and leptin during sexual maturation of a teleost fish. We investigated the effects of Kiss in the goldfish, Carassius auratus, on changes in KissRs, GnRH, three gonadotropin hormones (GTHs: GTHα, FSHβ, and LHβ), leptin mRNA, and activity, with in vivo and in vitro experiments.
Materials and methods
1. Study organism
For each experiment, female goldfish (n = 240; length = 8.1 ± 0.5 cm; weight = 15.2 ± 0.4 g; gonadosomatic index [GSI; gonad weight/body weight × 100] = 1.55 ± 0.08) were purchased from a commercial aquarium (Choryang, Busan, Korea). The fish were acclimated for 2 weeks in eight 300-L circulation filter tanks in the laboratory. The water temperature and photoperiod were 20 ± 1℃ and 12-h light : 12-h dark (lights on from 0700 to 1900), respectively. The fish were fed a commercial feed twice daily (at 0900 and 1700).
2. Kiss treatment and sampling (in vivo)
To investigate the effects of Kiss, fish were reared in 100-L circulating filter tanks in the laboratory and were anesthetized with 2-phenoxyethanol (Sigma, MO, USA) prior to injection. In all experiments, fish were administered single acute exposures by intraperitoneal injection of Kiss (metastin 45~54 amide; Sigma) dissolved in 0.1% saline solution at a volume of 10 μl g-1 body mass (BM).Exposure concentrations used were 0.1 and 0.5 μg g-1 BM. A sham group of fish was injected with an equal volume of 0.1% saline solution (10 μl g-1 BM). A control group of fish was untreated with a Kiss and 0.1% saline solution. After injection, pituitary, brain, liver, and plasma samples were collected from the fish at 0, 6, 12, 24, and 48 h. During the experimental period, water temperature and photoperiod were maintained at 20 ± 1℃ and 12-h light : 12-h dark, respectively.
All fish were anesthetized with 2-phenoxyethanol and decapitated prior to tissue collection. Pituitary, brain, and liver samples were removed from the fish, immediately frozen in liquid nitrogen, and stored at -80℃ until total RNA was extracted for analysis. The plasma samples were separated from blood samples by centrifugation (4℃, 10,000 × g, 5 min) and stored at -80℃ until analysis.
3. Culture of liver and pituitary cells and subsequent Kiss treatment (in vitro)
3.1. Pituitary cell culture
After the fish were anesthetized, the pituitary was dissected and placed in an ice-cold medium (pH 7.5) composed of 25 mM HEPES, 4 mM NaHCO3, 0.3% bovine serum albumin (BSA), 0.1% collagenase, 0.25 mg ml-1 fungizone, and RPMI medium containing antibiotics (100 U l-1 penicillin and 100 mg l-1 streptomycin; Penicillin-Streptomycin, Gibco, MA, USA). The pituitary was cut into 1~3 mm3 pieces, weighed, and placed in a 24-well culture plate (SPL Life Science, Korea) containing 1 ml of medium and incubated at 20 ± 1℃ for 1 day. Kiss was dissolved in an equal volume of 0.1% saline solution at the appropriate doses (0.1 and 1.0 μg ml-1) and added to the culture medium. The pituitary cells were cultured for 0, 6, 12, 24, and 48 h in an incubator at 20℃, 100% humidity, and 5% CO2. Following the incubation period, each sample was centrifuged (20°C, 10,000 × g, 15 s), and the supernatant was stored in individual microcentrifuge tubes at -80℃.
3.2. Liver cell culture
Livers were dissected and cut into 1~3 mm3 pieces, weighed, and placed in a 24-well culture plate. Under a sterile hood, the pieces were washed several times with culture medium solution (M199, Invitrogen, CA, USA). The liver pieces were added in equal amounts (approximately 50 mg) to each well of a 24-well plate and a total of 2 mL of fresh culture media was added. The liver pieces were allowed to acclimatize at room temperature for 2 h, at which time the indicated concentrations of Kiss (0.1 and 1.0 μg ml-1)
were added; an equal volume of distilled water (dH2O) was added to the control group. The liver pieces were cultured for 0,6, 12, 24, and 48 h in an incubator at 22°C, 100% humidity, and 5% CO2. Thereafter, each sample was centrifuged (20℃, 10,000× g, 15 s), and the supernatant was stored in individual microcentrifuge tubes at -80℃.
4. Quantitative PCR (QPCR)
QPCR was conducted to determine the relative mRNA expression levels of KissR1, KissR2, GnRH, GTHs (GTHα, FSHβ and LHβ), and leptin, using the total RNA extracted from the goldfish tissues. The primers for QPCR are shown in Table 1. These primers were designed for each gene using the Beacon Designer software (Bio- Rad, Hercules, CA, USA). Primer alignments were performed with the BLAST database to ensure the specificity of primers. QPCR amplification was conducted similarly to previous studies using a BIO-RAD CFX96TM Real-Time System (Bio-Rad). QPCR was performed in the following manner: 1 cycle of denaturation at 95℃ for 5 min, followed by 35 cycles of denaturation at 95℃ for 20 s, and annealing at 55℃ for 20 s. Each reaction was run in triplicate to confirm consistency. The experiments were duplicated with β- actin as an internal control. The efficiencies of the reactions were determined by performing the QPCR. All data were expressed as changes with respect to the corresponding β-actin-calculated cycle threshold (ΔCt) levels. The calibrated ΔCt value (ΔΔCt) for each sample and internal control (β-actin) was calculated as ΔΔCt = 2-(ΔCtsample - ΔCtinternal control).
5. Western blot analysis
The total protein isolated from the brain and pituitary was extracted using a T-PER® Tissue Protein Extraction Reagent (Thermo Fisher Scientific, Inc., Waltham, MA, USA), sonicated, and quantified using the Bradford method (Bio-Rad). A total of 25 μg of protein was loaded per lane onto Mini-PROTEAN® TGXTM Gels (Bio-Rad), and a protein ladder (Bio-Rad) was used for reference. Samples were electrophoresed at 180 V, and the gels were immediately transferred to a 0.2-μm polyvinylidene difluoride membrane (Bio-Rad) at 85 V for 3 min using the Trans-Blot® TurboTM Transfer System. The membranes were blocked with 5% milk in Tris-buffered saline (TBS) (pH 7.4) for 45 min and then washed in TBS. Thereafter, the membranes were blocked with 5% skim milk in 0.04% TBS with Tween (TTBS) for 45 min and subsequently washed in TTBS. The membranes were incubated with GnRH antibody (LRH- 13, a monoclonal mouse antiserum that recognizes most vertebrate GnRH forms; dilution, 1:5,000; courtesy of M.K. Park [Park and Wakabayashi, 1986]), followed by horseradish peroxidaseconjugated anti-mouse IgG secondary antibody (dilution, 1:5,000; Bio-Rad) for 60 min. In addition, membranes were incubated with a polyclonal rabbit antibody to GTHα antibodies (anti-goldfish GHα; dilution, 1:4,000; courtesy of M. Kobayashi [Kobayashi et al.,2006]), followed by horseradish peroxidase-conjugated anti-rabbit IgG secondary antibody (dilution, 1:5,000; Bio-Rad) for 60 min. The internal control was a β-tubulin antibody (dilution, 1:5,000; ab6046, Abcam, UK) followed by a horseradish peroxidase-conjugated anti-rabbit IgG secondary antibody (1:5,000; Bio-Rad) for 60 min. The bands were detected using WesternBrightTM ECL (Advansta Inc., Menlo Park, CA, USA) with a 30-s exposure and a Molecular Imager® ChemiDocTM XRS+ System (Bio-Rad). The membrane images were scanned with a high-resolution scanner, and the band density was estimated using a computer program (Image LabTM Software, version 3.0, Bio-Rad). The ratios of the internal control (β-tubulin) to the GnRH and GTHα for each concentration were calculated and plotted against the concentration of the internal control.
6. Plasma parameter analysis
The levels of plasma GnRH, FSH, LH, and 17β-estradiol (E2) were analyzed using the immunoassay technique with ELISA kits (GnRH [catalog no. CSB-E08810f; Cusabio Biotech Co. Ltd., China], FSH [catalog no. MBS035576; MyBioSource, USA], LH [catalog no. MBS283097; Mybiosource, USA], E2 [catalog no. MBS283228; MyBioSource], and leptin was analyzed using a Leptin ELISA kit [catalog no. CSB-EL012870FI; Cusabio Biotech, China]).
An anti-antibody specific to the antibody and antigen of the hormones (GnRH, FSH, LH, E2, and leptin) was pre-coated onto a microplate, followed by addition of 50 μl of plasma and 50 μl of HRP-conjugate. These were mixed well and then incubated for 1 hat 37℃. Following the last wash, any remaining Wash Buffer was aspirated or decanted off, and 50 μl of substrate solution was added to each well. The substrate solutions were then incubated for 15 min at 37℃ in the dark, during which time they changed from colorless or light blue and then to darker shades of blue. Following incubation, 50 μl of stop solution was added to each well, resulting in a color change from blue to yellow. The optical density of the solution in each well was then determined within 10 min using a microplate reader set to 450 nm.
7. Statistical analysis
All data were analyzed using the SPSS statistical package (version 10.0; SPSS Inc., USA). A two-way analysis of variance followed by Tukey's post-hoc test was used to test for significant differences in the data (p < 0.05). The values are expressed as the means ± standard error (SE).
Results
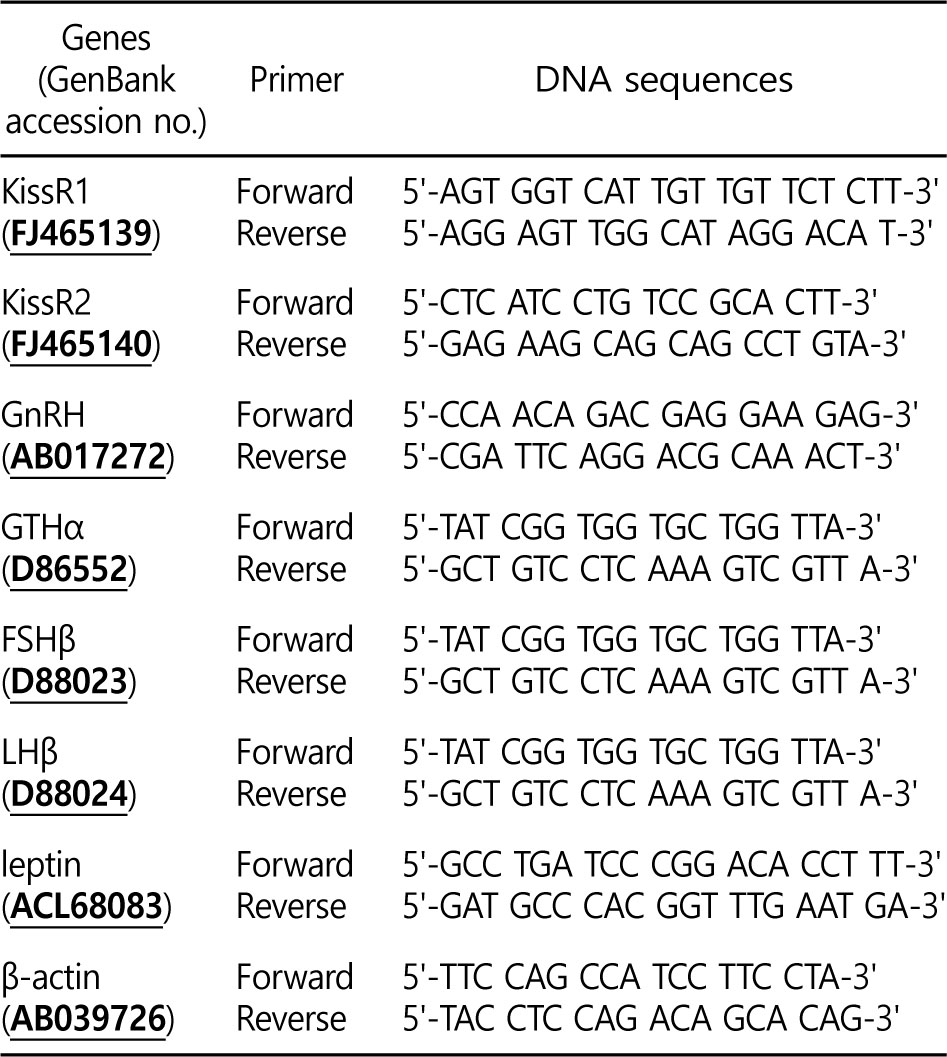
1. Time-course and dose-related effects of Kiss on KissR1 and KissR2 (in vivo)
Treatment with 0.1 μg g-1 and 0.5 μg g-1 of Kiss significantly increased the two types of KissR mRNA levels in the brain of the goldfish (Fig. 1). In particular, after the Kiss treatment, the level of KissR1 and KissR2 mRNA reached levels that were approximately 21.9- and 4.3-fold higher at 24 h than those in the control and sham, respectively (p < 0.05).
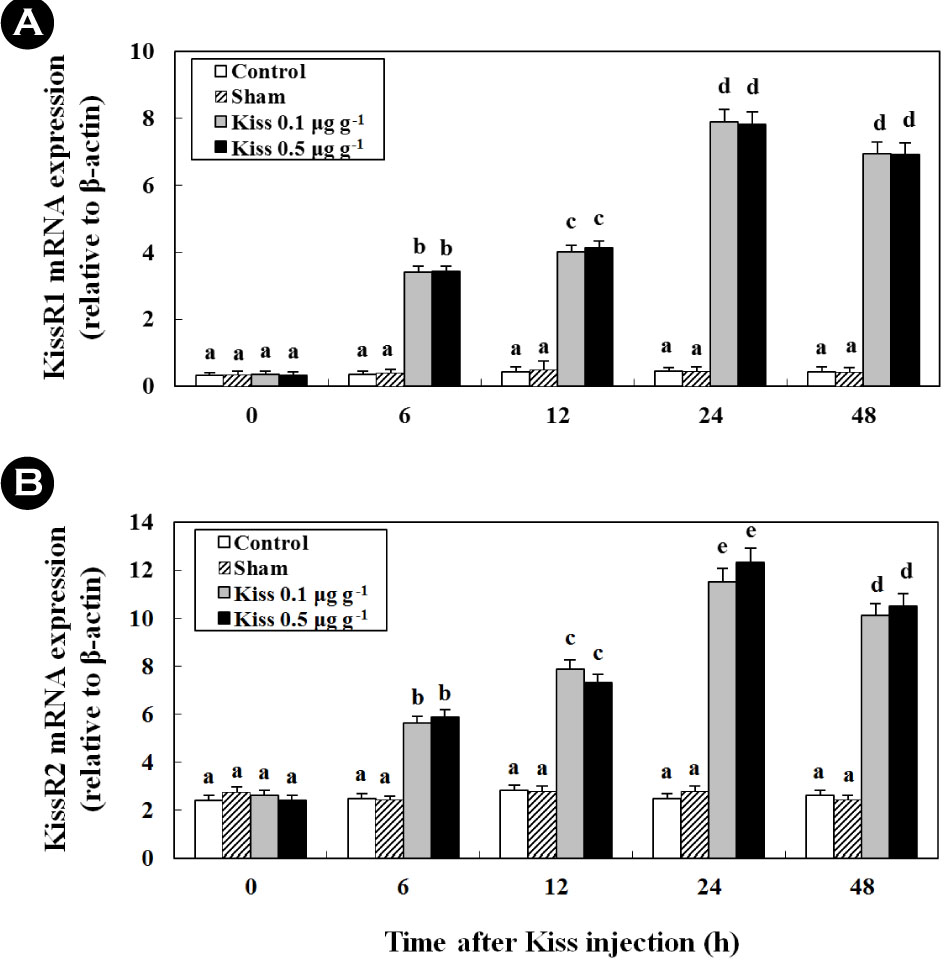
2. Time-course and dose-related effects of Kiss on GnRH (in vivo)
Following the Kiss injection, at 24 h, the expression of GnRH mRNA significantly increased and reached levels that were approximately 2.7-fold higher than those in the control (Fig. 2). Moreover, a western blot analysis revealed a protein with GnRH-specific immunoreactivity and a mass that corresponded to the predicted mass for goldfish GnRH (52 kDa; Fig. 2A). The expression pattern of the protein resembled the pattern of the GnRH mRNA expressed in goldfish brains. In addition, plasma GnRH levels in goldfish were 8.49 ± 1.58 ng ml-1 at the beginning of the experiment (Fig. 2C). Most notably, the GnRH levels increased to 4.59 ± 3.5 ng ml-1 and 42.58 ± 2.1 ng ml-1 at 24 h following treatment with 0.1 μg g-1 and 0.5 μg g-1 of the Kiss, respectively (p < 0.05).
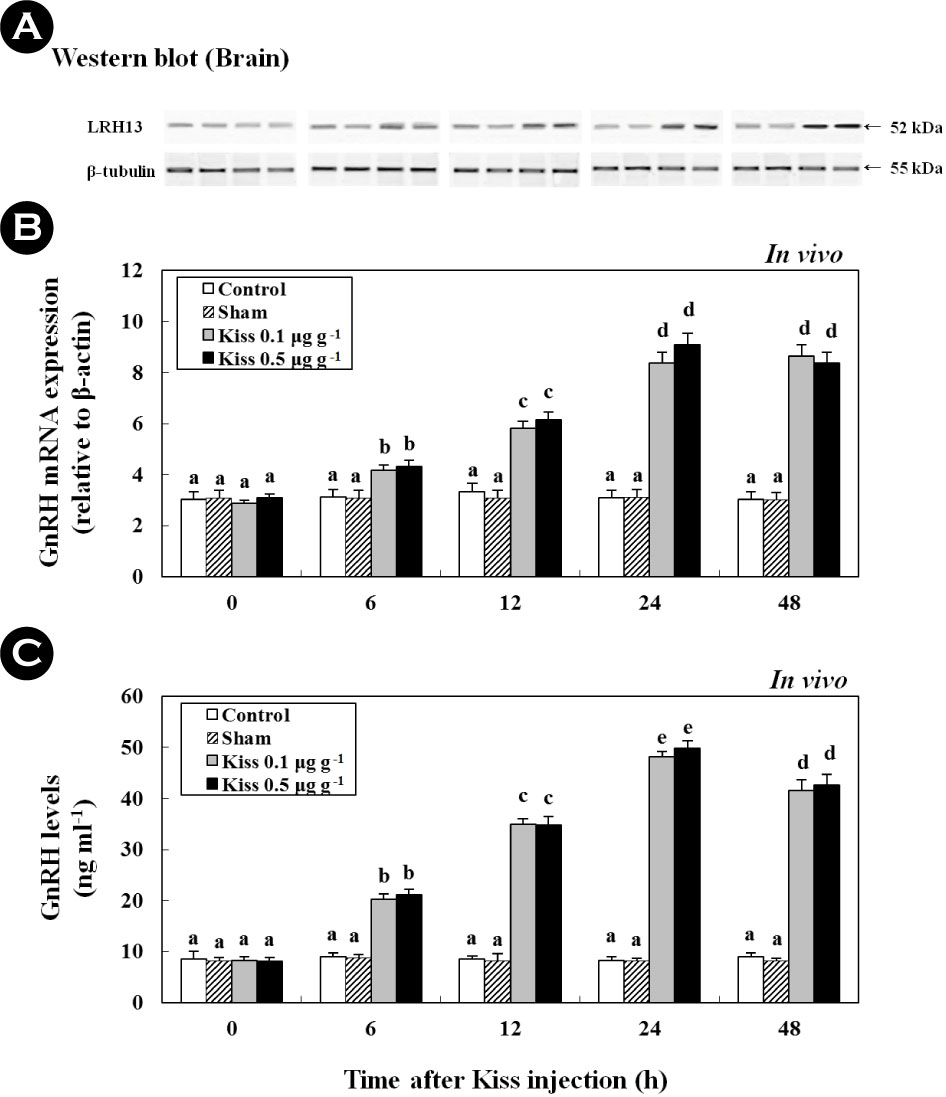
3. Time-course and dose-related effects of Kiss on GTHs (in vivo and in vitro)
Treatment with 0.1 μg g-1 and 0.5 μg g-1 of Kiss significantly increased the GTH (GTHα, FSHβ, and LHβ) mRNA levels in the pituitary of the goldfish (Fig. 3; in vivo). In particular, after the Kiss treatment, GTHα, FSHβ, and LHβ mRNA reached levels that were approximately 7.9-fold (Fig. 3B), 8.9-fold (Fig. 3C), and 6.3-fold (Fig. 3D) higher at 24 h than those in the control and sham, respectively. In addition, a western blot analysis detected a GTHα protein at a size that corresponded to the predicted size for goldfish (approximately 35 kDa), which exhibited similar mRNA expression across GTHα (Fig. 3A; p < 0.05).
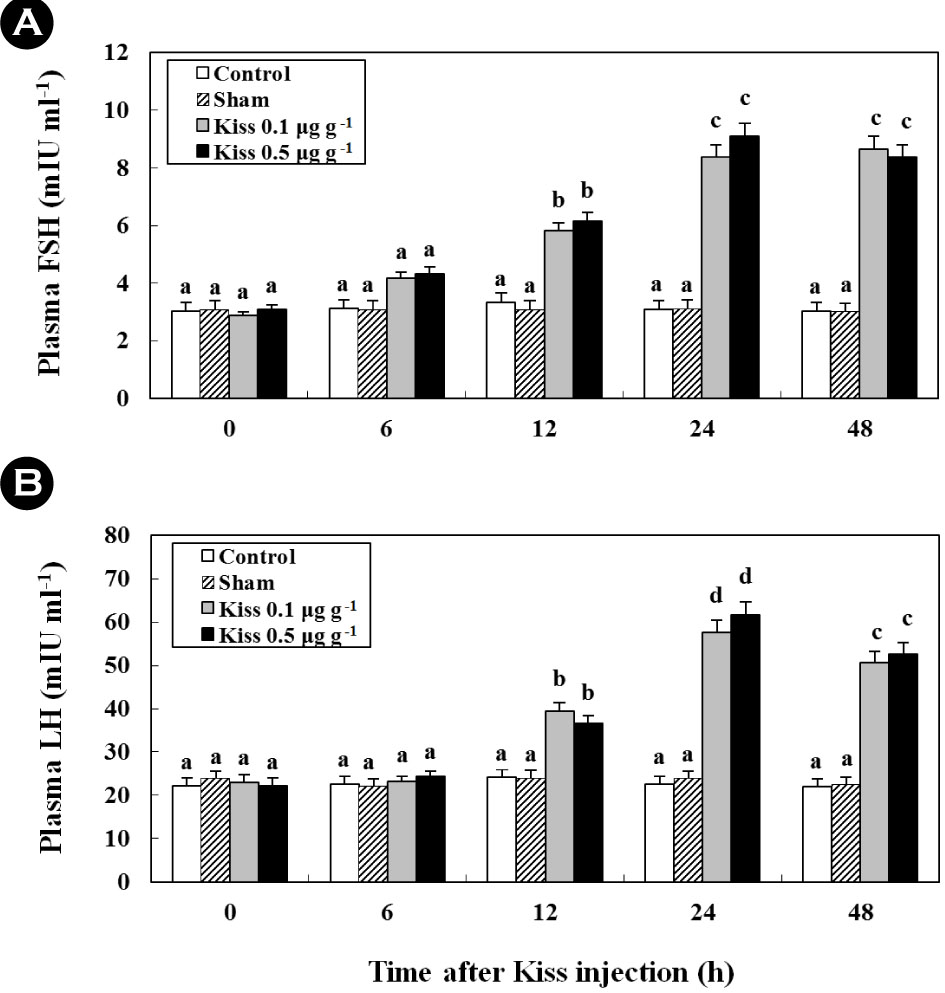
Following the Kiss injection, the plasma FSH and LH levels significantly increased and reached levels that were approximately 2.7- (8.4 ± 0.4 mIU ml-1; Fig. 4A; in vivo) and 2.3-fold (21.3 ± 1.5 mIU ml-1; Fig. 4B; in vivo) higher at 24 h than their controls, respectively.
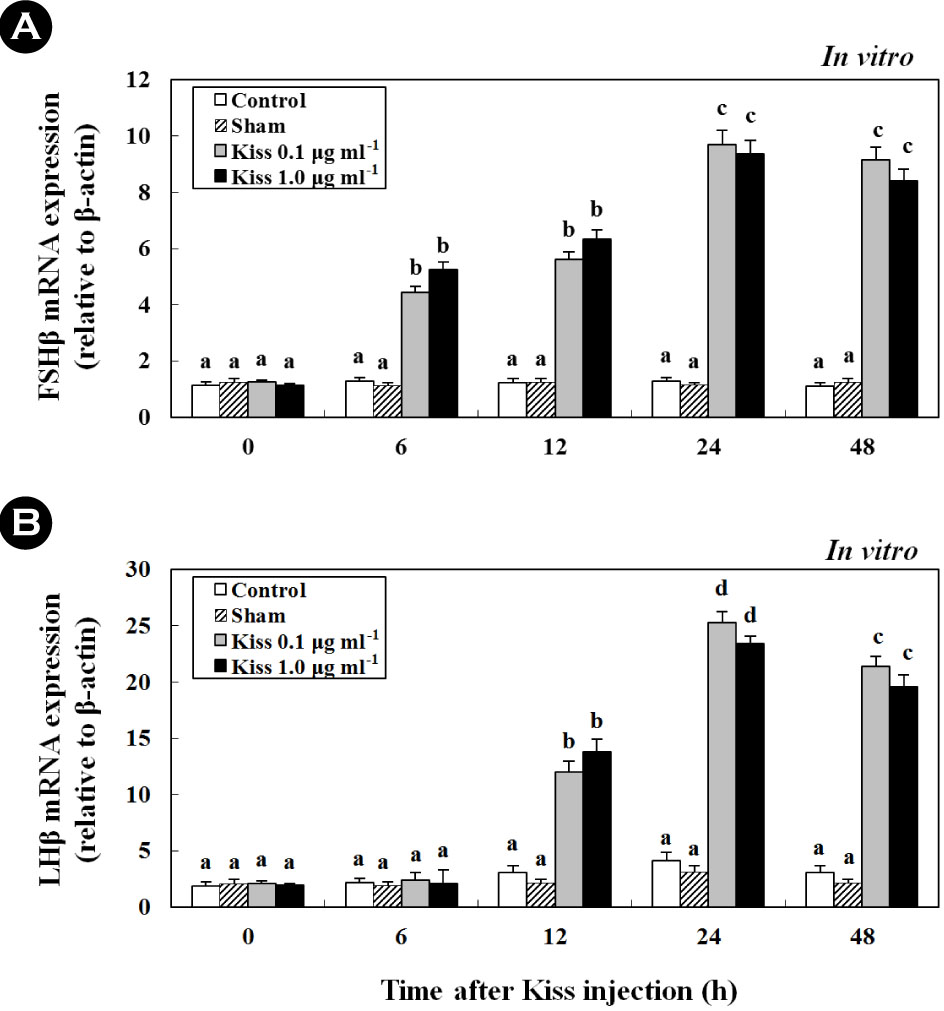
Furthermore, treatment with Kiss significantly increased FSH and LH mRNA levels in the cultured pituitary cells of female goldfish (Fig. 5A, B; in vitro). Additionally, at 24 h following Kiss injection, the expression of FSH and LH mRNA significantly increased and reached levels that were approximately 7.4- (Fig. 5A) and 10.3-fold (Fig. 5B) higher than their controls, respectively (p < 0.05).
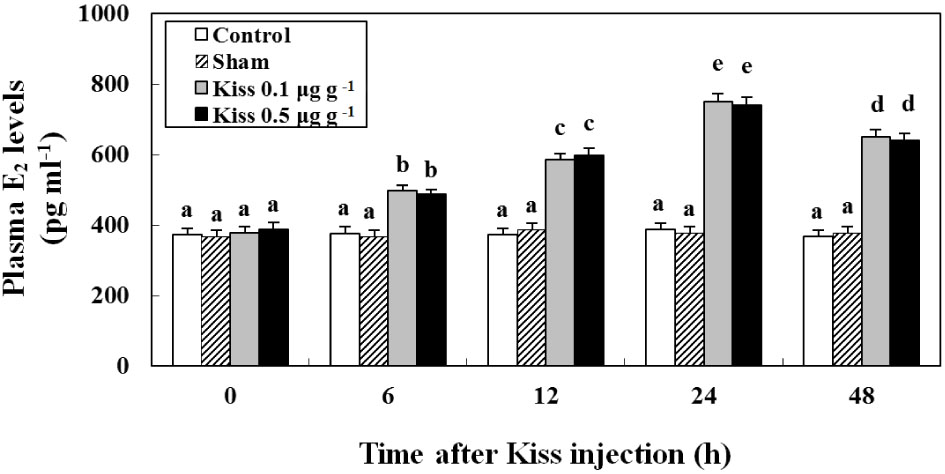
4. Time-course and dose-related effects of Kiss on E2 (in vivo)
The plasma E2 levels of female goldfish injected with Kiss are shown in Fig. 6.
The plasma E2 level was 372.5 ± 17.8 pg ml-1 at the beginning of the experiment. Most notably, the level of plasma E2 increased to 651.2 ± 20.1 pg ml-1 in fish treated with Kiss (p < 0.05).
5. Time-course and dose-related effects of Kiss on leptin (in vivo and in vitro)
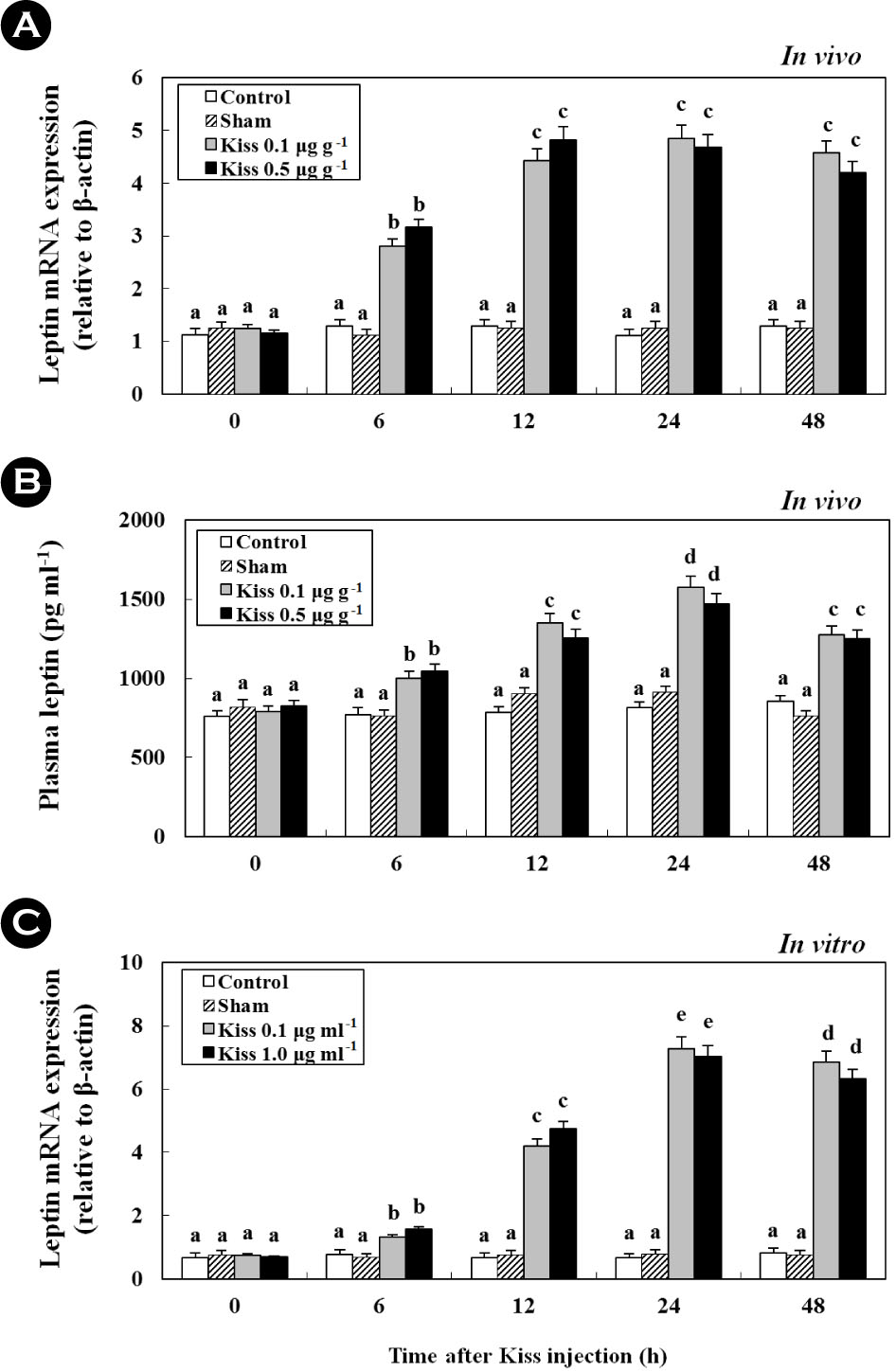
After treatment with 0.1 and 0.5 μg g-1 BM of Kiss, the expression of leptin mRNA reached levels that were approximately4.1-fold higher at 24 h than those of the control and sham (Fig.7A; in vivo). Similar to leptin mRNA expression in the liver, the plasma levels of leptin significantly increased to 1,274.3 ± 50.3 pg ml-1, approximately 1.6-fold that of the control (Fig. 7B; in vivo). Additionally, at 24 h following the injection, the expressionof leptin mRNA from the cultured liver significantly increased and reached levels that were approximately 9.2-fold higher than the control (Fig. 7C; in vitro) (p < 0.05).
Discussion
We investigated the effects of Kiss injections on the regulation mechanism of sexual maturation in goldfish, as assessed through the effects of Kiss on KissR (GPR54), HPG axis genes (GnRH and GTHs), leptin mRNA expression, and plasma GnRH, FSH, LH, and
leptin. We found that mRNA expression levels of the two types of KissR mRNA increased significantly with increasing processing time following Kiss injections in all experimental groups. In particular, the expression levels of Kiss1 mRNA increased significantly after the Kiss treatment and showed the greatest increase at 24 h. According to Alvarado et al. (2013), expression of both Kiss and KissR mRNA increases in the hypothalamus of female European sea bass, Dicentrarchus labrax, during oogenesis. In addition, we found that plasma GnRH levels increased with increasing processing time after Kiss treatment, but there were no significant differences between the different Kiss treatment concentrations. Zhao and Wayne (2012) and Nocillado et al. (2012) reported that an intraperitoneal injection of Kiss induced the secretion of GnRH in the medaka, Oryzias latipes, and yellowtail kingfish, Seriola lalandi. Tena-Sempere et al. (2012) reported that Kiss activated the HPG axis by stimulating the GnRH neurons through their receptor, KissR. Kiss and KissR play an important role in the physiological regulation of reproductive maturation; they control reproductive function and fertilization metabolism through the regulation of the secretion of gonadotropin hormones, the feedback actions of sex steroid hormones, and environmental signaling for the acceleration of the GnRH neuron until puberty (Tena-Sempere et al.,2012).
Therefore, we investigated the changes in GTHs mRNA and plasma E2 according to neuron expression levels of GnRH, the secretion of which was stimulated by Kiss. Expression of GTH mRNA and levels of FSH and LH also significantly increased with increasing processing time after Kiss injection. The results of this study were similar to those of previous reports wherein FSH and LH mRNA expression was reported to have increased after Kiss injections in the adult female zebrafish (Kitahashi et al., 2009) because of stimulation of the HPG axis.
Furthermore, we investigated whether Kiss directly affected the HPG axis. We observed the mRNA expression of FSHβ and LHβ of pituitary cells by Kiss treatment. Our results showed that the expression of FSHβ and LHβ mRNA increased significantly and was affected by Kiss concentration and duration of the period following treatment. Similarly, LH levels in the pituitary cells of goldfish (Chang et al., 2012) and European eel, Anguilla (Pasquier et al., 2011), increased following treatment with Kiss, and Kiss was shown to affect pituitary cells. Additionally, levels of plasma E2 increased with Kiss treatment. Therefore, we hypothesized that Kiss would interact with other steroid hormones secreted from the gonads. Our results were in agreement with a previous study (Kim et al., 2014) in which Kiss treatment regulated ER mRNA expression and the plasma E2 level, thus playing an important role in the sexual maturation of cinnamon clownfish, Amphiprion melanopus. We suggest that Kiss also affects final maturation of goldfish.
In teleosts, leptin is synthesized in the adipose tissue and secreted into the blood. Leptin plays roles in the synthesis of appetite suppressants and activation of HPG axis. It leads to increases in FSH and LH secretion and as such regulates sexual maturation and reproduction (Peyon et al., 2001, 2003; Frøiland et al., 2010). Liu et al. (2010) reported that high expression of the leptin receptor was observed in the hypothalamus and gonad of the zebrafish. Frøiland et al. (2010) reported that expression of leptin mRNA significantly increased at the beginning of sex maturation in the Arctic char, Salvelinus alpinus.
Additionally, mRNA expression and activity of leptin significantly increased with increasing processing time for both Kiss treatments (0.1 and 0.5 μg g-1). Similarly, Castellano et al. (2006) found that mRNA expression of Kiss, KissR, and LH increased after leptin treatment in rats. A previous study suggested that leptin receptors exist on cell membranes of Kiss neurons and therefore leptin combined with leptin receptors activates Kiss neurons and regulates the expression of Kiss. Furthermore, previous studies investigating the relationship between leptin and sex maturation found that leptin treatment increased LH and somatolactin in the sea bass (Peyon et al., 2001, 2003), and leptin treatment increased both FSH and LH in the cultured pituitary cells of rainbow trout (in vitro treatment; Weil et al., 2003). Therefore, similar to findings from previous studies, the expression and activity of leptin significantly increased with the interaction of Kiss and leptin, and Kiss and leptin promoted sex maturation of goldfish through activation of the HPG axis.
In summary, Kiss, which is located in the pre-optic area cells of the hypothalamus, stimulated the secretion of GnRH in female goldfish and played an important role in HPG axis activity. Moreover, using artificial Kiss treatments, we found that Kiss induced leptin expression, which is a hormone that affects the development of ovaries and promotes sexual maturation of goldfish.
Acknowledgments
This research was supported by the project titled 'Innovative marine production technology driven by LED-ICT convergence photo-biology', and by Korea Institute of Ocean Science and Technology (PN66370).
- References
-
3. Castellano JM, Navarro VM, Fernández-Fernández R, Roa J, Vigo E,Pineda R, Dieguez C, Aguilar E, Pinilla L, Tena-Sempere M. 2006. Expression of hypothalamic KiSS-1 system and rescue of defective gonadotropic responses by kisspeptin in streptozotocininduced diabetic male rats. Diabetes 55: 2602-2610.
-
14. Messager S, Chatzidaki EE, Ma D, Hendrick AG, Zahn D, Dixon J, Thresher RR, Malinge I, Lomet D, Carlton MB, Colledge WH, Caraty A, Aparicio SA. 2005. Kisspeptin directly stimulates gonadotropin-releasing hormone release via G proteincoupled receptor 54. Proc Natl Acad Sci U S A 102: 1761-1766.
-
15. Nocillado JN, Biran J, Lee YY, Levavi-Sivan B, Mechaly AS, Zohar Y, Elizur A. 2012. The Kiss2 receptor (Kiss2r) gene in Southern Bluefin Tuna, Thunnus maccoyii, and in Yellowtail Kingfish, Seriola lalandi - functional analysis and isolation of transcript variants. Mol Cell Endocrinol 362: 211-220.
-
18. Peyon P, Vega-Rubín de Celis S, Gómez-Requeni P, Zanuy S, Pérez-Sánchez J, Carrillo M. 2003. In vitro effect of leptin on somatolactin release in the European sea bass (Dicentrarchus labrax): dependence on the reproductive status and interaction with NPY and GnRH. Gen Comp Endocrinol 132: 284 -292.